Shallow Investigation: Bacterial Meningitis
This report consolidates a shallow investigation into Bacterial Meningitis—its effects and importance in global health, the current tractability and cost-effectiveness of leading interventions, and an evaluation of the overall promise of the cause. I estimate this report to be a result of about 60-70 hours of research and writing. This research was conducted as part of the Cause Innovation Bootcamp fellowship, with constant guidance from Dr. Akhil Bansal.
Summary:
Meningitis is an inflammation (swelling) of the protective membranes covering the brain and spinal cord. It is commonly associated with infections (e.g. bacterial meningitis, viral meningitis), but it can also have non-infectious causes. The most common symptoms include fever, headache, sensitivity to light, and neck stiffness; in most cases, meningitis is treatable by addressing the underlying cause e.g. treating the causative infection.
Bacterial meningitis is important from a global health perspective—it ranks 40th on the current list of diseases in terms of total DALYs lost.
4 strands of bacteria cause 50% of all meningitis-related deaths, namely meningococcus, pneumococcus, Haemophilus influenzae and group B streptococcus—all of which are vaccine-preventable.
GBS (Group B Streptococcus) ranks 6th on the list of causes leading to DALYs lost in the age-group 1-10.
Bacterial meningitis is heavily concentrated in the African Meningitis belt, consisting of regions in 26 countries stretching from Senegal in the West to Ethiopia in the East, and incidence is inversely related with socio-demographic index (SDI).
Bacterial meningitis does not seem to be neglected -
Important steps seem to have been taken already to counter meningitis on a global scale; including WHO’s comprehensive report on “A Global Plan to Defeat Meningitis by 2030”.
While the important interventions seem tractable, they seem to be less neglected than a lot of other interventions in global health, reducing their counterfactual value.
Important interventions that could yield (relatively) high cost-effectiveness seem to be -
Installing an integrated disease surveillance and response (IDSR) system for monitoring meningitis, and
Advocating for the acceleration of the GBS vaccine development.
Major uncertainties: The interventions are still ‘moderately’ promising; for ex. If someone is uniquely positioned to accelerate GBS vaccine trials/distribution, or complete broad educational initiatives about infant healthcare/precautions preventing neonatal transmission, this might on the margins be a promising thing to do. On another note, the counterfactual neglectedness is low primarily due to WHO’s commitments in its “Roadmap to defeating meningitis by 2030”—if not followed up/held, the counterfactual value of another route to addressing meningitis could increase significantly.
I. Importance
Meningitis is an inflammation (swelling) of the protective membranes covering the brain and spinal cord. It is commonly associated with infections e.g. bacterial meningitis, viral meningitis, but it can also have non-infectious causes. The most common symptoms include fever, headache, sensitivity to light and neck stiffness; in most cases, meningitis is treatable by addressing the underlying cause e.g. an infection[1].
Meningitis, depending on the specific pathogen (virus, bacteria, fungi etc.) is often communicable and usually transmitted through close contact. Meningitis can also be passed on by mothers to their infants, and in fact, bacterial meningitis in infants is most commonly caused by the Group B Streptococcus pathogen (GBS), passed down in the peripartum period ( thebirthing process). Since meningitis attacks the membranes of the spinal cord and the brain, the most worrisome symptoms relate to the nervous system and include—hearing loss, seizures, brain damage etc. Bacterial meningitis can be especially fatal if untreated, with 1 in 10 patients dying[2]. Even in the case of receiving treatment, meningitis can lead to long-term sequelae[3], most often causing focal neurological deficits, cognitive impairment, hearing loss, multifaceted mental and emotional harm, and even death.
Meningitis remains of great importance within the Global health sphere. It ranks 6th in the list of diseases causing DALYs for children under the age of 10[4], as published in a systematic analysis in 2019. The mortality rate is heavily concentrated in ages 1-20, and both the incidence and mortality rates peak in the age group of 1-5, steadily declining after. Recent trends [5]have shown spikes in the incidence of meningitis from pre-pandemic levels, and frequent outbreaks within gay or bisexual men. [6]The UN in June of 2021 came up with the ‘Roadmap to defeat meningitis by 2030’[7], which seeks to eradicate bacterial meningitis epidemics, reduce vaccine-preventable cases by 50% and deaths by 70%.
Meningitis-related deaths in 2016 amounted to 318,400, which was a decrease of 21% from 1990. However, incidence in the same duration went up 12.8%, from 2.5 million to 2.82 million. In 2016, meningitis caused 1.48 YLDs, compared with 21·87 million DALYs. As a result, it seems that most of the burden from meningitis comes from the deaths that it causes, rather than long-term disability[8].
Mortality and incidence are inversely related to SDI (Socio-demographic index) [9]. The highest mortality rates and incidence rates were found in the peri-Sahelian countries that comprise the African meningitis belt, with six of the ten countries with the largest number of cases and deaths being located within this region. The “meningitis belt” in Africa includes parts of at least 26 nations, stretching from Senegal in the West to Ethiopia in the East.[10]
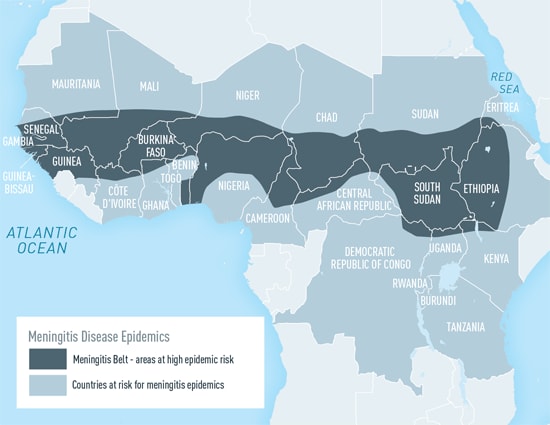
The primary interventions (further examined in this report) include the PsA-TT vaccine[11], which was developed to combat Neisseria meningitis (MenA) epidemics in Africa, the MenACWY [12] vaccine which counters the A, C, W, and Y strands of bacterial meningitis, and improvements to the surveillance and response systems to cases of meningitis. Other promising interventions (not examined due to lack of credible data) include raising awareness and running educational initiatives about meningitis (and broadly meningococcal disease) (done by orgs like National Meningitis Association), increasing uptake of a pneumococcal vaccine during pregnancy[13], and creating administrative policies in schools for meningitis prevention.[14]
This report restricts its findings to bacterial meningitis because -
Viral meningitis is fairly common and usually resolves within a week or two. It rarely causes a critical condition and/or needs medication.
Just 4 bacteria are responsible for more than 50% of death caused by all-cause meningitis—meningococcus, pneumococcus, Haemophilus influenzae and group B streptococcus—all of which are vaccine-preventable except the GBS, for which a vaccine is currently in development.[15]
Most credible research available on interventions such as vaccine rounds, effectiveness, burden etc. is heavily centred on bacterial meningitis, with no recent and credible evidence for the global burden on other-cause meningitis and their interventions (such as. fungal meningitis).
II. Neglectedness:
Bacterial meningitis has recently gotten the spotlight in both academic and policy discourse—in 2018, a set of health economists and doctors in the UK completed a cohesive economic analysis of existing vaccines with policy recommendations[16], and organisations like GAVI and NMAUS are actively working in the meningitis belt to deliver interventions cost-effectively and educate families on prevention techniques. Perhaps most importantly, a meeting in May 2017 of more than 50 global experts, led by the Meningitis Research Foundation in collaboration with WHO, resulted in a global roadmap to defeat meningitis by 2030—a plan that required broad global commitments to strengthen systems for prevention, treatment, and surveillance.
However, there is a wide scope for research into meningitis that could help shape the future of the field, with some warranting immediate concern.
There have been no RCTs or cohort studies conducted on empirical therapy for treating meningitis. Interventions like antibiotics, therapy, and or aftercare remain unexamined for efficacy and/or cost-effectiveness.
Risk factors for bacterial meningitis and meningococcal disease outbreaks aren’t fully understood, making prediction of epidemics unlikely[17]. The WHO also mentions “increasing lab capacity and infrastructure to detect potential epidemic pathogens” as a priority in its report. The most recent outbreak in Florida (2021-2023), primarily affecting gay and bisexual men, was caused by a vaccine-preventable strand of meningitis.
Vaccination strategies for specific strains and countries in order to achieve herd immunity have not been developed, leading to no clear health policy directions that can be taken up by affected nations.
The most common cause of fatal bacterial meningitis in infants is Group B Streptococcus—a bacteria for which a vaccine still hasn’t been developed. Given the highest mortality lies in the 1-5 years age group, this seems to be a highly neglected area.
Answers in these key uncertainties could significantly update the immediate promise in Bacterial meningitis as a cause area. Research in the field seems necessary, but recent trends in the burden of bacterial meningitis clearly show that the cause area isn’t neglected by academics, policy-makers, or on-ground institutions (such as non-profits). The next section shows how, except for i) the development of an active surveillance program, and ii) expediting the development of the GBS vaccine, none of the research gaps seem to warrant urgency when compared to other neglected issues in global health.
III. Tractability (Possible interventions and their theories of change)
There are many different approaches and interventions to reduce the burden of meningitis; the table below summarizes the main interventions that were investigated in this report, which a priori seemed the likeliest to be cost effective and promising. The cost-effective analyses are attached at the end of the report.
For an easier read, the following table is also colour-coded and pasted on the sheet with cost-effectiveness analyses, attached at the end of the post.
| Intervention | Outputs | Outcomes | Quality of evidence | Approximate Cost-effectiveness | Overall promise |
| Improving surveillance and response systems | Build the medical and healthcare infrastructure to closely monitor and immediately respond to meningitis | Improve data collection and response system | Moderate; several studies show the infrastructure building to be cheap, + expert opinion (mentioned as a key intervention by academics and WHO) | Unclear with current evidence; possibly high - there’s some data for meningitis specifically, but other comparable bacterial diseases show such surveillance systems to be cost-effective* | Between moderate and high, leaning high; also mitigates the neglected fields of epidemic forecasting/controlling. Builds robust infrastructure for the future |
| Accelerate development of GBS vaccine via policy advocacy | Early production/distribution of an approved vaccine against GBS. | Reduction of GBS cases/stillbirths + Improved maternal/neonatal healthcare, + potentially earlier herd immunity against GBS. | Low; the evidence of policy advocacy is pretty non-transitive (a success story in another disease would not necessarily mean success in this one). The guiding force here is expert opinion (1Day Sooner). This study also shows that the awareness of GBS as a public health concern is lowest amongst health policy makers. | Between moderate and high; (possibly very high) although completely dependent on the cost (which is unknown and subjective to campaign), studies on a 60% efficacious vaccine show cost-effectiveness between $676-$2390 per DALY, although these can be considered lower bounds**. | Between moderate and high; awareness amongst policymakers seems low, and organizations like 1DS or GAVI or NMA seem uniquely placed to advocate for trials/funding of the GBS vaccine at a very small cost, and the incidence of the disease is large enough that acceleration of even a year or two is highly valuable. |
| Distribute the existing MenACWY vaccine | Protection against strands A, C, W, and Y of the meningococcal bacteria. | Achieving herd immunity against popular strands of bacterial meningitis, possibly avert epidemics | Moderate; (efficacy is well documented, but costs are estimated using just 2 studies from 2 countries with sample size <30) | $43.44/DALY saved (this is probably a upper bound, the conjugate nature of the vaccine may lead to herd immunity quicker) | Moderate; (each strand has decreased in burden (and therefore importance) in the past decade) |
| Distribute the existing PSa-TT vaccine | Protection against the serogroup A meningococcal bacteria. | Achieving herd immunity against popular strands of bacterial meningitis, possibly avert epidemics | High; (efficacy studies seem well-conducted, and costs are derived using GAVI’s 15 year progress) | $206.99/DALY saved (accounts for DALYs caused by serogroup A only, vaccine may as well have overlapping benefits against other strands) | Low; serogroup A isn’t as neglected anymore, the vaccine is arguably cost-ineffective at the margin compared to other interventions |
Accelerate development of GBS vaccine by funding clinical trials* *based on very rough estimates, not at par with the credibility of other estimates in this table—caveat elaborated in the next section | Early production/distribution of an approved vaccine against GBS. | Reduction of GBS cases/stillbirths + Improved maternal/neonatal healthcare, + potentially earlier herd immunity against GBS. | Low; found just one study that said clinical trials are a challenge impeding the GBS vaccine development.No GBS-specific data found, Success probability and Costs are estimated from studies concerning other bacterial diseases. | Between low and moderate; erring low. There are no studies that explicitly state/estimate how new trials would lead to significantly better results/acceleration of vaccine development. | Between low and moderate; Although tractability seems high, given WHO’s recent emphasis on GBS, trials don’t seem counterfactually neglected. It is also hard to gauge the importance without studies estimating how new trials would directly accelerate vaccine development. |
| Education initiatives about meningitis transmission and good practices | Educate families about meningitis prevention techniques | Increased prevention, regulation, and treatment of meningitis cases. | Low—expert opinion (mentioned in the WHO document and some government recommendations) + working of organizations like the NMAUS | Unclear; all significant benefits are secondary and there are no cohort studies to examine long-term changes in health/income etc. No RCTs/cohort-studies found that estimate the burden of meningitis education directly averts. | Between low and moderate; most secondary benefits (increased income, increased school attendance etc.) have other interventions that directly affect them—so the promise doesn’t seem incredibly high solely on second-order benefits. |
| Distribute existing Antibiotics | Protect against bacterial pathogens of meningitis | Treating patients with meningitis effectively | Low—there seems to be no standard prescription of antibiotics that apply to a set of bacteria—the exact meds (and hence costs) seem dependent on the strand, duration, age etc. of the patient. No clear standard dose, and no study conducted on efficacy. | Unclear; probably comparable to vaccines, or likely even less cost effective since delivery isn’t publicly financed, and there is a possibility of patients not finishing their doses with time. Also comes with a significant diagnosing cost—since antibiotic courses have to be specific to patient (pathogen, other health conditions etc.) | Low to moderate, leaning low. |
Interventions I found but didn’t dive deeper in:
Funding clinical trials for the GBS vaccine—although the report includes a cost-effectiveness analysis for this intervention, there is inadequate data on the specifics of the GBS vaccine—major uncertainties in possible efficacy of the vaccine, doses required for immunization, room for funding amongst vaccine developers, the projected expedition caused by additional funding etc. render this analysis superficial. Any novel information on any of the aforementioned factors could be vital in cost-effectiveness.
Pneumococcal vaccination during pregnancy—the evidence for this was medium with one cohort study conducted—the cost effectiveness was unclear since even if costs were assumed to be about the same as other vaccinations, the benefits were unclear as the tests found differing results in terms of effectiveness. The overall DALY burden of neonatal meningitis is centred in GBS, for which a vaccine is currently in the works—which demonstrably should be much more effective.
Research on key areas—as shown in the ‘Neglectedness’ section, there are multiple research gaps within the field that would prove valuable if found answers for. But since promisingness depends on the researchers who take these questions up (their counterfactual value etc.), and since there is no way to estimate the costs/benefits directly, I didn’t dig deeper.
Research on school administrative policies on meningitis prevention—this intervention is based off just one study conducted in Southern Nigeria[18] but since the burden is heaviest in the schoolgoing age category, research into prevention policies can be super valuable. The intervention also seems costless, except the time it would take schools to formulate, communicate and regulate these policies. This one is moderately promising as it targets the right population.
Caveats and Notes on promising interventions
I. Integrated Disease Surveillance and Response (IDSR) and its cost-effectiveness:
Probably much more cost-effective than it seems due to overlapping benefits of IDSR for all other diseases and preventing further epidemics. Given the per capita cost of establishing the system is $0.07, and the system may have overlapping benefits for all diseases, the intervention seems very cost-effective. Such surveillance systems may have a strong use case for antimicrobial resistance and novel pathogen detection (especially those with pandemic potential).
Although the study indicates presence in Burkina Faso, Eritrea, and Mali, it is unclear if such a system is robust in these countries, and whether it is available in other countries in the Meningitis Belt—but it remains a topic of consideration in the WHO report of defeating meningitis, so it is assumed that this space is fairly neglected.
It is unclear what the ‘improved completeness of surveillance’ means—therefore the analysis just provides 2 conservative estimates of 1 and 5 percent improved DALYs saved per case—by means of reaching patients quicker, preventing epidemics, improved aftercare, preventing sequelae etc. Even by these conservative numbers, the benefits seem cost effective.
The neglectedness of the IDSR is pretty unknown. The study is based on 3 countries within the meningitis belt—Burkina Faso, Eritrea, and Mali—which gives accurate estimates of the costs, but also shows that the IDSR is already present and operational in some countries that this report seeks to target. However, the WHO has still listed the creation of an active surveillance system (albeit not specifically an IDSR) as one of its 5 ‘pillars’ in its “Defeating Meningitis by 2030: A global road map” report.
The specific costs of the IDSR are based on just one study, and it is noteworthy that the study doesn’t provide a detailed breakdown of the utility of such an IDSR (i.e, it is possible that the IDSR studied was for a specific disease, was
II. Policy advocacy for the Group B Streptococcus vaccine:
The studies cited assume a mean cost range between $10 - $30 - however, traditional costs for comparable vaccines (such as the Psa-TT shown in the CEA sheet) can be as low as $3.49 including delivery costs; given the vaccine is manufactured and delivered at scale. This drives up the possible cost effectiveness.
The productivity losses would arguably be much lower for pregnant women or infants (perhaps a very slightly longer recovery period etc.) but the indirect costs seem significantly lesser for the GBS vaccine when compared to the MenACWY or Psa-TT, further driving up its cost-effectiveness.
The costs of advocacy and the probability of success are intertwined. Paradoxically, spending more on the advocacy might have an exponentially larger impact on influencing policy than a poorly funded one; and so i) lesser costs do not necessarily make the intervention more cost-effective, and ii) the cost-effectiveness heavily hinges on the relationship between costs and the probability of success.
The cost-effectiveness also heavily hinges on the efficacy of the vaccine itself. The study from South Africa assumes 60% efficacy, swinging cost-effectiveness between the $676 to $2390 range, while the above reasons are enough to believe that the Cost-effectiveness might be much higher.
The average delivery costs are taken from a study that measured the costs during the Covid-19 pandemic—so the data is i) recent and ii) relevant to the rolling out a new vaccine (just like the GBS vaccine would be). However, an important caveat is that the indirect costs are completely unknown. In the case of Covid-19, the indirect costs were low (for ex. the side-effects of the vaccine didn’t warrant extended leaves from work), but the PsA-TT vaccine had an average of 21 days of recovery period (thereby large indirect/opportunity costs). As such, the indirect costs of the GBS vaccine are unknown, and could weigh heavily on the cost-effectiveness calculations.
The model also runs on the assumption that the GBS vaccine would only require a single dosage of the vaccine—this could easily be a false assumption. Multiple doses would have subsequent impact on indirect costs incurred, delivery strategies, costs, and vaccine efficacy if dosage is incomplete—all of which drive down cost-effectiveness.
Conclusion:
Overall, this report finds that Bacterial Meningitis has some promising avenues of investment in the forms of GBS vaccines (via the advocacy route, but also potentially by funding clinical trials). However, it seems like a battle more than half won. Interventions dealing with most other strains of bacterial meningitis are not nearly as cost-effective, and the burden for these has been steadily declining. The cost-effectiveness and spillover benefits of installing an IDSR system seem to make it a compelling competitor against some of the most cost-effective interventions in Global health. From a broad view, important agents like GAVI, Pfizer and the WHO are actively involved in tackling Bacterial Meningitis, which makes it less likely that they are neglected enough for the Effective altruism community to look deeper into.
It is noteworthy that there are a lot of research bottlenecks that might further inform this conclusion. The most obviously urgent research field remains the Group B Streptococcus vaccine, but other fields remain germane, such as i) understanding patterns of pathogens causing meningitis epidemics, ii) conducting randomised evaluations of the effects of therapy, aftercare, and antiobiotics, iii) creating improved surveillance systems that help coordinate health policy across the Meningitis belt, and iv) researching into specific age-based interventions (prenatal vaccination, school administrative policies). Answers from all these questions can significantly update the promise of bacterial meningitis as a cause area.
Cost-effectiveness analyses here: CEA—Bacterial Meningitis
Thanks for this nice to see Global health stuff again on the forum (definitely a minority of posts :D ). Nice shallow investigation it was good to get a bit of an update on the situation here.
A couple of queries/criticisms
I’ve got some issues with your IDSR analysis. I basically, agree with your points 2 to 5, but point 1 feels loose with unlikely assumptions. “ISDR” is such a vague concept I think it would have been worth spending a couple of paragraphs outline what that actually is and how it worked in this test case.
“Probably much more cost-effective than it seems due to overlapping benefits of IDSR for all other diseases and preventing further epidemics. Given the per capita cost of establishing the system is $0.07, and the system may have overlapping benefits for all diseases, the intervention seems very cost-effective. Such surveillance systems may have a strong use case for antimicrobial resistance and novel pathogen detection (especially those with pandemic potential).”
I don’t understand why you make the leap to ISDR seeming “very cost-effective” t based on the low cost and potential overlap for other diseases. Having seen these kind of vague programs in Uganda, the net benefit might be close to zero, so it’s dangerous to make assumptions of any impact at all. This kind of system will never detect a new pathogen, and unless it actually changes behaviour of prescribing health professionals (unlikely), then I don’t see how it would prevent antimicrobial resistance.
Out of interest as wellwhat triggered you to focus on Bacterial Meningitis? It seems to me instinctively like a fairly well understood problem, with vaccines already available for Meningitis and in development for Group B strep. You might have found something promising in your research but it seems unlikely.
Interesting analysis. I learned a bunch of things.
One comment: the 1⁄10 rate is the mortality of treated patients. The mortality rate of untreated bacterial meningitis is much closer to 100%.[1] This means the number-needed-to-treat for antibiotics goes from above 10 to between 1 and 2. How would an order of magnitude difference here change your analysis?
Reliable recent numbers are hard to come by, probably because ~all conclusively diagnosed patients in western countries are treated or diagnosed posthumously. There is an NEJM paper citing numbers (from the pre-antibiotic era) between 70% and 100% for the different pathogens.