Kidney stone pain as a potential cause area
This is a cross-post from my Substack (original post here).
Note: At the Effective Altruism Global: San Francisco conference in 2017, Prof. Will MacAskill implored the audience to “keep EA weird”. As the EA movement grows, it’s important to keep EA’s original spirit of exploration alive. To help do that, I’m planning to write several articles on potential new — and weird — cause areas.
Effective altruists want to figure out how to do the most good per dollar spent. “Good” is often cached out in terms of deaths prevented or quality-adjusted life years saved (QALYs). QALYs attempt to adjust life-years for different states of health. However, the very method of QALY calculation, which typically involves surveys asking about trade-offs, might have some blind spots. For instance, if a disease state is rare, than most likely the requisite survey data for it has never been collected. The surveys themselves may have blind spots too. Consider these points from Andrés Gómez Emilsson (emphasis mine):
“Someone described the experience of having a kidney stone as ‘indistinguishable from being stabbed with a white-hot-glowing knife that’s twisted into your insides non-stop for hours’. It’s likely that the reason why we do not hear about this is because (1) trauma often leads to suppressed memories, (2) people don’t like sharing their most vulnerable moments, and (3) memory is state-dependent (you cannot easily recall the pain of kidney stones .. you’ve lost a tether/handle/trigger for it, as it is an alien state-space on a wholly different scale of intensity than everyday life).”
As Daniel Kahneman describes in his book Thinking Fast and Slow, the remembering self is different than the experiencing self. People have trouble describing and conceptualizing extreme events, either positive or negative. People also don’t like thinking about extreme negative events generally, whether they experienced them or others did. I personally sometimes notice my brain flinching away when thinking about kidney stone pain, even though I haven’t experienced it myself.
In the first part of this post I’ll go over the evidence for extreme pain events. Then, I’ll focus on kidney stones. The main reason for focusing on kidney stone pain is that over the past two years I’ve worked off-an-on on automated deep learning based software for detecting and measuring kidney stones in CT scans (see my paper in Medical Physics). So I have some expertise on the subject. Currently I am working with a radiologist at Massachusetts General Hopsital who is an expert on stone disease, Prof. Avinash Kambadakone.[1]
Background—suffering focused ethics
“In my opinion human suffering makes a direct moral appeal, namely, the appeal for help, while there is no similar call to increase the happiness of a man who is doing well anyway.”
“Instead of the greatest happiness for the greatest number, one should demand, more modestly, the least amount of avoidable suffering for all.”— Karl Popper, The Open Society and Its Enemies (1945)
The idea that we should focus on eliminating suffering over increasing pleasure is intuitive to many people. See this recent Twitter poll from Robin Hanson:
So, I don’t think I need to spend much time here convincing people that reducing suffering should take precedent over increasing happiness. Note what I have in mind here is what is called “weakly-negative utilitarianism” which is quite different than pure negative utilitarianism, which focuses only on eliminating suffering. Readers interested in diving further into these topics should check out Lukas Gloor’s essay “The Case for Suffering-Focused Ethics”.
Background—long-tailed distributions of pleasure and pain
“Finding scalable treatments for migraines, kidney stones, childbirth, cluster headaches, CRPS, and fibromyalgia may be extremely high-impact.” — Andrés Gómez Emilsson
A typical pain scale used by doctors. The patient is asked to name a number or point on the scale.
Studying pain is notoriously difficult, and trying to compare pain qualia (experiences) is even more so. Most commonly, a doctor may ask a patient to rate their pain on a ten point scale like the one shown above. But is the difference going from level four pain to level five the same as the difference between level nine and level ten? Furthermore, what determines the bounds of the scale? We all have a clear idea what 0⁄10 pain is from experience, but what is 10⁄10 pain? Clearly, “worst pain possible” is going to require some imagination, and different people will have different conceptions of what that means. Anecdotal evidence suggests people often report experiencing “radically new” levels of pain beyond what they formerly thought was possible. So, the 10 point scale will have a lot of variance in it across people since they will be different conceptions for what 10⁄10 pain is. There are probably other sources of variance too— I am not aware of this variance being studied directly (ie with electric shocks).
In his 2019 EA Forum post “Logarithmic Scales of Pleasure and Pain: Rating, Ranking, and Comparing Peak Experiences Suggest the Existence of Long Tails for Bliss and Suffering”, Andrés Gómez Emilsson argues that there exist tail experiences in both pain and pleasure. Extreme pain events, although rare, could play a rather large role in the utilitarian calculation effective altruists attempt to carry out. The existence of tail experiences also makes binning pain experiences into 10 buckets with 10 being the worst is a bit wonky. If tail experiences are 10x or 100x more painful than common forms of pain, then most pain should concentrated between 0-1 on the 10 point scale — but that’s not how the scale is used.
The most convincing data to support the idea of tail experiences in pain come from preliminary survey data that Andrés presents. The dataset is very small, but a pretty clear finding is that when people think about their worst experience, many describe it as significantly worse than the second worst. Here’s the data:
This clearly looks like a heavy-tailed distribution. It would be very instructive to do more surveys like this, in particular with more people who have experienced kidney stones, cluster headaches, and other ailments. This seems like very low-hanging fruit for EA.
The three medical issues that came up highest for pain in Andrés’ survey were kidney stones, cluster headaches, and childbirth. Among the respondents, 9⁄93 (9.7%) mentioned kidney stones. This data is roughly in line with the life-time risk for getting a symptomatic kidney stone and suggests that among those who are unfortunate enough to have a kidney stone, for most of them it will result in one of the most painful experiences in their life. Cluster headaches have a 100x lower prevalence than kidney stones (~1/1000 instead of ~1/10) but among suffers pain from cluster headaches occurs much more frequently on average than kidney stones, possibly 100x more so. So integrating pain over time, it’s possible cluster headaches may be a bigger problem.
Here’s some other evidence that Andrés presents for the existence of tail experiences:
The firing activity of groups of neurons follows a lognormal distribution, a type of distribution that contains a long tail of rare extreme events. The intensity of the sensory stimuli is communicated via the firing rate of the afferent nerves. It seems parsimonious to assume that internally in the brain encodes stimuli intensity via firing rate (although there are other plausible scenarios, such as having different neurons or groups of neurons represent different levels of stimuli intensity (population coding)).
The Schmidt pain index (for insect bites), the Scoville scale (for hot peppers), and the KIP scale (for cluster headaches) have all been described by their creators and users as being exponential in nature.
Personal reports from drug users. People who take psychedelics are often surprised when taking a higher dose or a different substance dramatically increases the intensity of the experience beyond what they were expecting.
The burden of stone disease is rising
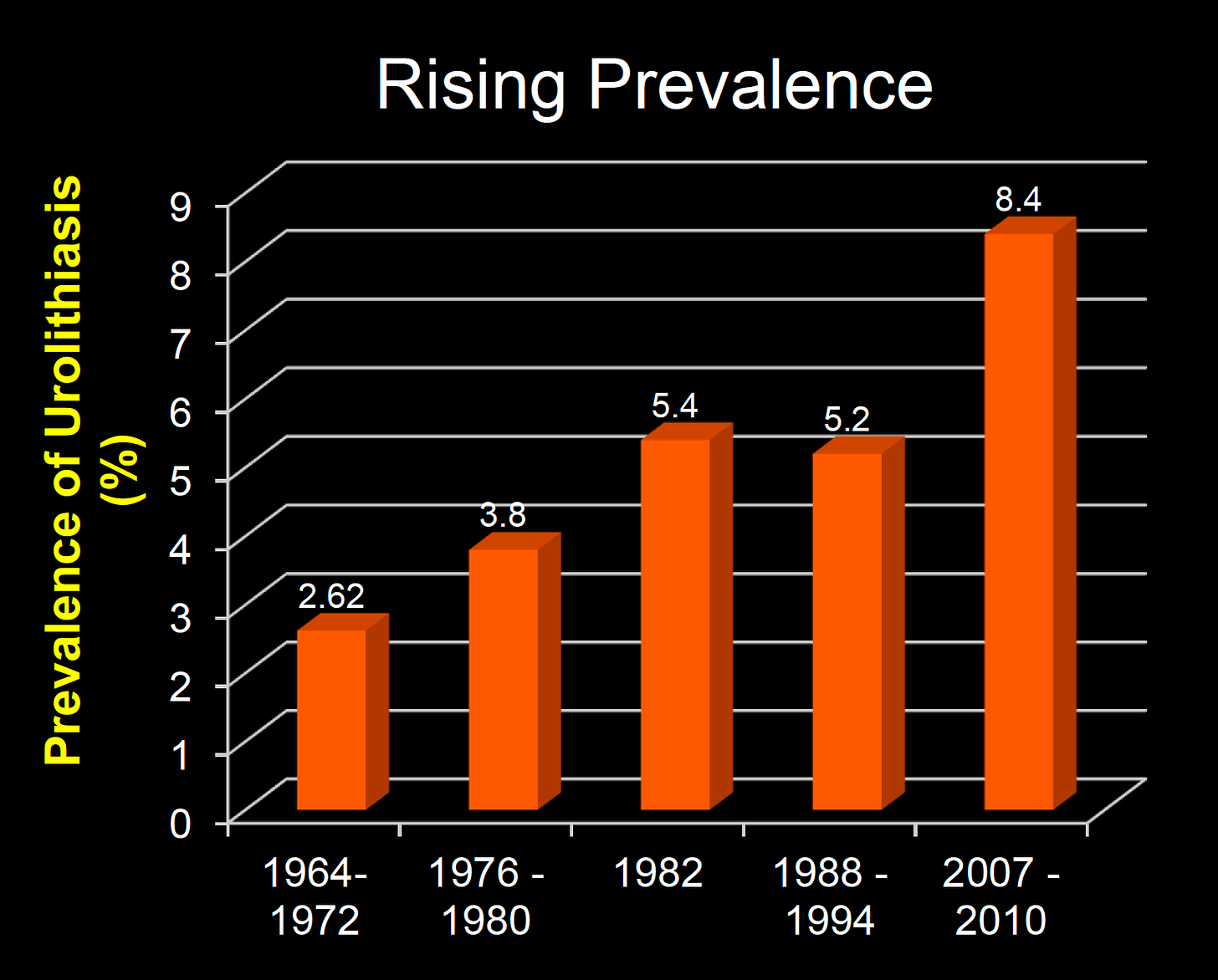
The latest data indicates that in the United States people have a lifetime risk between 15-20% of having a urinary tract stone.[2] These numbers have been increasing over the years, so much so that prevalence studies from more than a decade ago are out of date. Many factors might explain these increases. Some notable factors that increase risk are salt consumption, dehydration, the consumption of animal products, and high protein diets. Kidney stones also have a high rate of re-occurrence. Over half of stone-formers will have another stone within five years.
A 2005 study estimated that the total direct and indirect costs of kidney stone disease were approximately $4.5 billion in 2000 in the United States.[3] The same authors estimate that a 75% effective intervention that that costs less than $300 per patient per year would be cost effective at reducing health care expenditures. They note that a shift away from expensive medications towards low cost treatment modalities such as increased water intake and lemon juice could increase cost effectiveness.[4]
Technologies for screening exist
Currently, people usually don’t learn they have kidney stones until they experience pain, typically flank pain on their side. This leads them to consult a doctor or go to the emergency room, where diagnostic scanning will be done to confirm the presence of kidney stones and decide on the best means of treatment. Kidney stones are the 9th most common cause for emergency room visits. From a suffering reduction approach, we need a way to screen patients before the pain starts to flare up. Another point is that the potential for painful obstruction etc is proportional to the max diameter of the stone. It may even be that pain of passing a stone goes up exponentially with the stone diameter. If we think of the stones as spherical (actually a crude approximation but illustrative here) then the amount of material on the surface of the stone goes as the diameter^2. So reducing diameter is easier when the stones are small. So early treatments should yield more bang per buck.
Several imaging techniques exist to screen for kidney stones—kidney-ureter-bladder (“KUB”) X-ray radiographs, ultrasound, MRI, and CT (colloquially called “CAT scanning”). A nice summary of the pros and cons of these different techniques is shown in this table:
The technique with the highest sensitivity is CT followed by MRI, which doesn’t involve any radiation dose but is roughly three times as expensive. Only CT is useful for detecting small stones (< 4 mm) and uric acid stones (which comprise about 15% of stones and are transparent on radiographs).[5]
For screening purposes, CT and ultrasound are only two viable options. The main issue with CT is radiation doses, which increases cancer risk to a non-trivial degree. However, low dose screening protocols have been developed and validated (I won’t bother my readers with technical details here, although I personally find them fascinating). The bottom line is that screening protocols now exist where the radiation exposure is on par with a KUB radiograph (about 1.5 mSv).[6] Adoption of these protocols has been slow, however. The effect of low dose radiation on cancer risk is somewhat controversial but 1.5 mSv given to people with an age around 50 would increase their lifetime cancer risk by about 1 in 10,000. In other words, you would get one new cancer for about every 10,000 scans performed. This is pretty non-trivial and further Fermi estimates are needed here to understand benefits vs risks.
How many kidney stones might we detect early via CT scanning? Potential kidney donors typically undergo a CT scan to screen for potential counter-indications. Studies show that about 7.5% of potential donors have previously undiscovered kidney stones.[7] Some transplant centers allow donors with kidney stones to donate, others do not. Many people get CT colonographies around age 60. When a CT colonography is performed, the radiologist is responsible not just for analyzing the colon but also for doing due diligence on the rest of the scan. In my work at NIH, I looked at 5,381 CT colonography scans that had reports available. Among those, 755 had keywords suggesting the that a kidney stone was found (15 %). [8]These scans were all from ~10 years ago, so current numbers might be a bit higher.
Unfortunately, my impression is that tiny stones may be downplayed in reports by radiologists due to concerns about ‘incedenalomas’ from CT scanning consuming too much of limited healthcare money and resources. Another point is that doctors often don’t recommend treatments for tiny stones because they assume “they will likely pass on their own”. From what I have been told, passing even tiny stones can be excruciatingly painful. To doctors, tiny stones are “not clinically significant”. Yet from a suffering reduction perspective we should consider that if we can treat tiny stones and can dissolve them in place then the patient can avoid the pain of having to pass the stone.
How do stones form?
Two things are required for stone formation—supersaturation of some dissolved substance and a nucleation site. For such a pervasive condition, it’s remarkable how recent most of the science is on it. Stone nucleation dynamics, genetics (stone disease is 4-50% heritable) and the role of bacteria (discussed below) all remain poorly understood. Calcium salt deposits on the tip of the renal papilla are believed to serve as nucleation sites for calcium-based stone formation. These deposits, called Randall’s plaques, result in slightly higher X-ray attenuation on CT, so theoretically they can be detected using CT techniques.
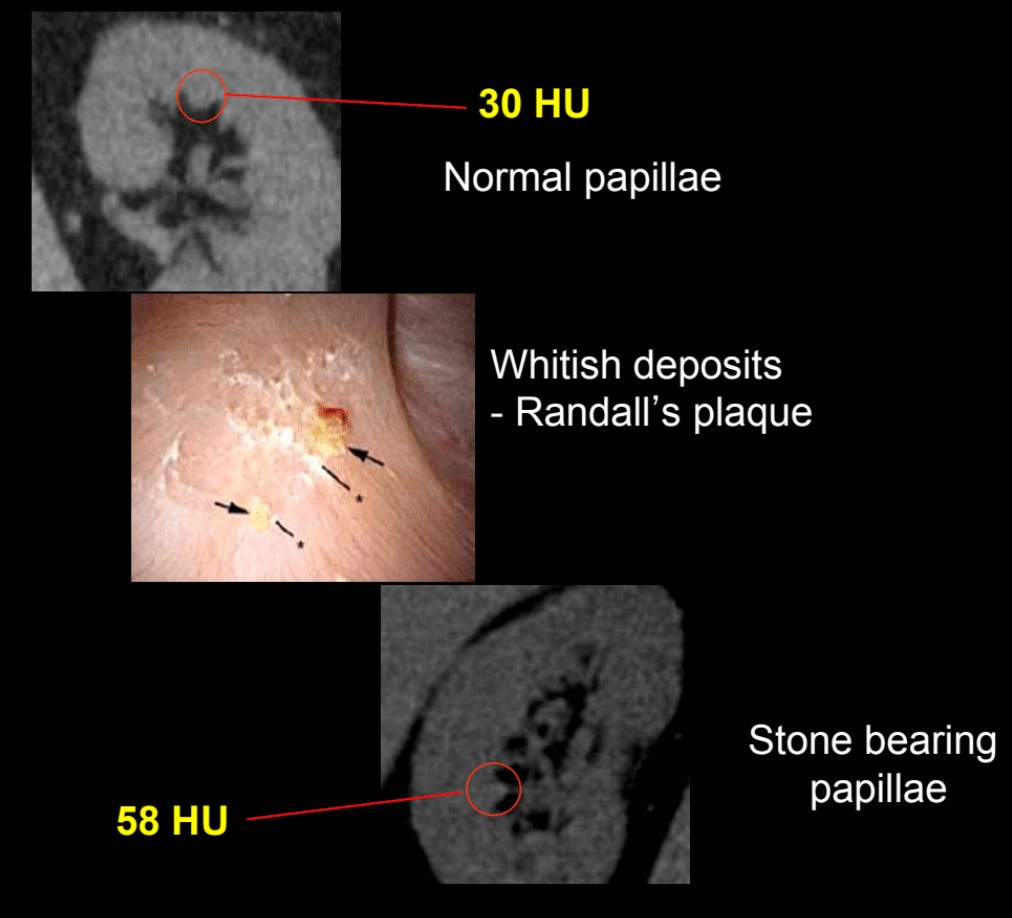
However, this is currently very challenging. A 2013 study found that studied this concluded that current CT technology cannot do it. I suspect that if we used the high resolution CT protocols for cardiac CT to analyze a single kidney instead of the heart, detecting Randall’s plaques may be quite feasible. This could be a future area for research.
Another interesting thing is that Oxalobacter formigenes, a bacteria discovered in 1985, appears to play an important role in breaking down oxalate. There is some preliminary work showing a correlation between the presence of this bacteria and decreased risk for calcium oxalate stones. Antibiotic use is also correlated with higher risk for kidney stone disease, and antibiotics may kill this bacteria. In the future, bacterial treatments may be possible, but the science here is still in its infancy — again, more research needed!
So you have a tiny kidney stone.. now what?
There are not a lot of large high quality studies on interventions for preventing kidney stones or kidney stone growth.[9] This points to a larger bias in medicine towards treating disease rather than preventing it in the first place. Still, it seems fairly well established that juices that are high in citric acid help prevent stone formation. Fresh squeezed lemon and lime juice have the highest concentration of citric acid, but people do not typically drink lemon and lime juice straight from the fruit — it is typically diluted first. A meta-review concluded that orange juice is best for preventing kidney stones, followed by lemon juice. On the flip side, Vitamin C, also found in fruits, increases oxalate levels, which contributes to the chance of getting calcium-oxalate stones, the most common type of stone. Other high-oxalate foods include spinach, almonds, and cocoa. Instead of sugary-juices, it is much healthier to consume sugar-free alternatives or just take potassium citrate in tablet form, which is very cheap. Lowering salt consumption and raising water intake are two other relatively easy things to do. There also exist prescription drugs like thiazide diueretics, but they don’t seem to be radically better than the simpler treatments already mentioned and may incur uncomfortable side effects. Uric acid stones, which comprise 10-15% of stones and can be distinguished somewhat well on CT scans, are known to be very easy to treat with drugs.
Finally, I don’t want to steal too much of his thunder here, but Andrés Goméz Emilsson has been doing some interesting work investigating the herb Phyllanthus niruri which is known as Chanca Piedra (“stone breaker”). See this podcast clip:
Andrés says he plans to publish a report on this later this year.
Conclusion
The main take-away here is that more research is needed. Most research on kidney stone disease has focused on treatments, leading to sophisticated and well-validated treatment techniques such as percutaneous nephrolithotomy, using catheters to blast the stones with lasers, placement of stents to help kidney stones to pass, and shock wave lithotripsy. Much less research has gone into understanding stone formation and growth dynamics, risk factors, early detection methods, and preventative therapy.
Here are some promising areas for future research:
Do more surveys on kidney stone pain and frequency. (Anectdotal reports suggest a small minority of people with kidney stones pass a large number of stones with frequencies as high as one per week.)
Development of better neuroimaging biomarkers for pain (fMRI, EEG, fNIRS), to move away from reliance on self-reports and bolster the case for tail pain states. (Side note : the Black Mirror episode Black Museum explores a doctor who uses an EEG device to feel the pain of patients. Hopefully this won’t be necessary to more rigorously compare pain states).
Better scanning tech for screening (lower dose CT, dual-energy CT for stone type, lower-cost MRI, AI+ultrasound).
More studies to validate and compare low-cost treatments for both prevention and removal of small stones (increased water intake, potasium citrate, Chanca Piedra). Ideally RCTs.
Cost effectiveness calculation / Fermi estimation of screening + treatment.
Acknowledgements
I want to thank Andrés Goméz Emilsson for a very helpful chat and for reading an early draft of this post.
Appendix—Weber’s law
How exactly do we perceive pain? A 1947 study using a 500 Watt heat lamp is somewhat instructive here. Using a lens, they focused a beam of heat onto their subjects foreheads. They wanted to understand if the perception of pain scaled linearly with the intensity of the light. However, they couldn’t just ask the subjects directly how much pain they were experiencing, because there is no standard reference scale for pain. The researchers carried out their experiment using the method of “just noticeable differences”. Essentially, this method involves increasing the stimuli until the subject reports a difference. They found that the subject’s ability to distinguish two different stimuli was inverse proportional to the strength of the stimulus, in line with an idea called Weber’s law. Interestingly, the researchers hit a limit around when the intensity of the heat source was above 500 millicalories / second / cm^2 — subjects could not distinguish intensities higher than this level. It appears the researchers maxed out the firing rate of the pain receptors (nociceptors) in the skin.
Some people think that Weber’s law goes against the idea that there can exist extreme pain events. The most straightforward interpretation of Weber’s law is that the brain applies something like a log() function to the input its receiving and it is this log() of the intensity that is rendered into our subjective experience (or qualia). Andres argues against this by noting an issue with the just-noticeable-differences method—it may be that the the intensity is rendered linearly, but then our ability to discriminate between those two sensory experiences goes logrithmically. This sort of makes an intuitive sense—a very strong sensory experience may overwhelm us and make it harder for us to do a fine-grained discrimination. The example Andres gave to me once is that if you are experiencing two large fiery hot flaming suns in your head it may be much harder to distinguish a 10% difference between them compared to two tiny softly glowing orbs.
However, I don’t find Andres’ argument that convincing. It seems more parsimonious that the that the conscious rendering of a stimuli would be proportional to the rate of firing of some neuron(s), and that our ability to distinguish between two firing rates should also be directly proportional to the rate (for instance being able to distinguish differences of +/-1%).
Secondly, it’s pretty well-accepted at this point that the brain does apply something like a log() function to render some inputs, and there are other methods beyond just-noticeable differences that back this up. This is most obvious for brightness. The sun is 398,110 times brighter than a full moon, but it doesn’t feel that much brighter. This is partially because our eye’s pupils dilate at night to let more light in and other adaptation factors, but the biggest reason is that our brain applies something like a log() function to the input.
The bigger issue with Weber’s law is that it doesn’t always apply. In fact, for electric shock the situation is the the reverse! (see figure blow) Could kidney stones be like electric shocks? Could the pain of a stone grow exponentially with the diameter of a stone? We just don’t really know.
- ^
For a deep dive into medical imaging and kidney stone disease see Prof. Kambadakone’s 2010 paper in Radiographics, the leading pedagogical journal for radiology.
- ^
Hill, Alexander J., et al. “Incidence of Kidney Stones in the United States: The Continuous National Health and Nutrition Examination Survey.” Journal of Urology, 207 (4), Apr. 2022, pp. 851–56. This papers study used 2007-2010 NHANES data and estimated that “19% of men and 9% of women will be diagnosed with a kidney stone by the age of 70”.
- ^
Saigal, Christopher S., et al. “Direct and Indirect Costs of Nephrolithiasis in an Employed Population: Opportunity for Disease Management?” Kidney International, vol. 68, no. 4, Oct. 2005, pp. 1808–14.
- ^
Kurtz, Michael P., and Brian H. Eisner. “Dietary Therapy for Patients with Hypocitraturic Nephrolithiasis.” Nature Reviews Urology, 8 (3), Mar. 2011, pp. 146–52.
- ^
Regarding the determination of uric acid vs non uric acid stones via CT—most studies on the accuracy here use stones inserted into “phantoms” that mimic the human body. Roughly speaking a trained clinician using CT can distinguish uric acid stones from non-uric acid with an accuracy around 40-60%. Distinguishing uric acid stones is important because even large uric acid stones can usually be treated with drugs (oral dissolution therapy) rather than requiring surgery or other invasive methods. Dual-energy CT (DECT) technology can distinguish the two types with near 100% accuracy. About 5-10% of hospitals now have so-called “premium” scanners which have dual energy capability, but DECT techniques have not diffused into clinical practice very much.
- ^
Planz, Virginia B., et al. “Ultra-Low-Dose Limited Renal CT for Volumetric Stone Surveillance: Advantages over Standard Unenhanced CT.” Abdominal Radiology, 44 (1), Jan. 2019, pp. 227–33.
- ^
Kim, Irene K., et al. “Incidental Kidney Stones: A Single Center Experience with Kidney Donor Selection: Kidney Stones and Donor Selection.” Clinical Transplantation, 26 (4), July 2012, pp. 558–63.
- ^
Elton, D. C., et al. “A deep learning system for automated kidney stone detection and volumetric segmentation on non-contrast CT scans.” Medical Physics. 49 (4) pgs 2545-2554.
- ^
Uribarri, Jaime. “The First Kidney Stone.” Annals of Internal Medicine, vol. 111, no. 12, Dec. 1989, p. 1006.
- Pain relief: a shallow cause exploration by (27 Jan 2023 9:55 UTC; 85 points)
- The Quest for a Stone-Free World: Chanca Piedra (Phyllanthus niruri) as an Acute and Prophylactic Treatment for Kidney Stones and Their Associated Extreme Negative Valence by (18 Feb 2025 0:08 UTC; 76 points)
- Monthly Overload of EA—June 2022 by (27 May 2022 15:48 UTC; 62 points)
So right after posting this I found a report called “Problem area report: pain” published by the Happier Lives Institute. (It seems they have taken the report off their website but you can find it on archive.org)
They say this: “We considered investigating other pain-related problems, but decided not to: … Kidney stones and trigeminal neuralgia – although very painful conditions, they are not as painful as cluster headaches or as common as advanced cancer.”
It’s not clear to me that cluster headaches are much more painful than kidney stones. Really what needs to be done is more Fermi estimation. Data on kidney stone pain frequency and duration seem non-existent. Doing the estimate would require some digging through personal reports online and possibly conducting surveys. I have other stuff I want to write so I pushed out this post without going down that rabbit hole.
Hi Dan. Thanks for referencing our report, which can be found at www.happierlivesinstitute.org/report/global-priority-pain
Hi Dan—great post, you’ve clearly put a lot of thought and research into this. I read the whole thing.
I’m a (predominantly) emergency doctor so the title of your post caught my eye. I can certainly confirm that stone-related pain brings even the most stoic amongst us through the doors of the ED to seek out help!
There were a couple of points that came to mind reading your post (and please feel free to take or leave them as you will, they are just preliminary thoughts and I’ve not put my own research into it, these are just my thoughts and my thoughts with my anecdotal doctor’s hat on):
Pain vs Suffering: As you’ve alluded to, pain can be quite unwieldy in its characterization and description. It’s also hugely tied to emotions, expectations, and outcomes. Whilst kidney stones are objectively and subjectively incredibly painful, I’ve often found that a good explanation of the cause of the pain and the assurance that it WILL pass with time is very reassuring and so the overall suffering attached to that pain experience is less say than the emotional suffering I’ve seen in patients with chronic intractable pain or pain that has no physical attributable cause (sometimes called functional pain). I do agree though that suffering attached to stones that don’t just pass and the operations etc. that might need to come after that can be unpleasant (interestingly and again anecdotally stent-related pain seems to be as bad if not worse than the stone itself and those things can stay in for months!). I just wonder whether it’s overall a big enough burden of suffering amongst the other forms of pain/suffering to warrant rigorous efforts to alleviate/prevent it.
Regarding small stones being downplayed: I don’t know that they’re necessarily downplayed (even if found as an ‘incidentaloma’ - for those not familiar: something that pops up on a scan that you weren’t looking for; from incidental), but for small stones not causing pain or obstruction, we don’t do anything about them straight away, yes (because they might pass and because ‘doing’ things in medicine is always a trade-off of doing harm vs benefit ie doing stent surgery (in the absence of something to make it go away otherwise) without good cause confers a lot more suffering than an asymptomatic stone!).
Radiation stigma: Again, this is a combination of reason and intuition but on the balance of all things considered (ie knowing the disease process, its likelihood etc), would I have a CT scan for a screening test at present? No. Regardless of how low the radiation is, as a young woman, it’s a cumulative risk I’ll avoid if I can. I think this way for patients too. I guess there’s different thinking with age and benefit; mammograms as a screening modality for breast cancer would be an example. Also obviously, US or MRI would remove the radiation risk but then you run into cost and access issues (also a consideration for CT scans) and the need for skilled sonographers.
I guess I say the above with the idea that in this space, the preventative approach seems more promising to me in terms of its cost-effectiveness and scalability than the diagnostic or therapeutic approaches. I’m also generally excited about the advances in medical imaging and the intersection with AI that are coming about!
Anyway, thanks again for your post, it was interesting for me to read.
Hi,
I’m just seeing your comment now for some reason. This is super helpful.
Regarding your first point (pain vs suffering), that’s pretty interesting and makes sense. I would just note that the degree to which people can detach from painful experiences varies. Regarding suffering from operations and stents, I have heard the same thing about stents, and that is something we would have to factor in, I think, to a Fermi estimate of the amount of suffering that could be alleviated with early interventions for kidney stones. I wonder if someone could invent a stent that actually diffuses a bit of anesthetic around it while it is in (my understanding is the stents are typically only temporary in place).
Regarding the second point “Regarding small stones being downplayed:”. So, after writing this I looked into it a bit further because I was interested in whether an AI application assisting in early detection might be high value. The idea that radiologists miss tiny stones is only my personal guess. I have only seen 1-2 examples of this, when I was running a system I developed for stone detection and it found stones in CT colonography scans that were not mentioned in the report.. but those 1-2 examples only surfaced after running on over 6,000 scans.
Regarding how what happens with tiny stones: data on this subject is very scarce, but it seems most tiny stones resolve on their own without major symptoms (?). It’s really not very clear. I found one paper which covers this question, although it doesn’t directly study it. Looking at CT scans for CT colonography they found that 7.8% of patients (all middle aged adults) had asymptomatic stones. They then found that only 10% of patients with asymptomatic stones were later recorded as having symptoms over a variable follow-up interval that extended to a maximum of 10 years. So it seems the tiny stones don’t cause symptoms… but maybe it takes longer than 10 years before they start to manifest symptoms.. Probably having a tiny stone puts you at massively higher risk for a symptomatic stone event later in life. There’s very little data on this question or about stone growth dynamics across lifespan in general.. it’s not something that’s very easy to study. Basically, scanning people with CT just to monitor their stone size is absurd, so to study this we have to mine historical scans and then try to find follow-up scans to see if the same stones are still there, and compare their volume (which is a bit tricky to do accurately when the scan parameters change). This is an application for deep learning based automated stone segmentation algorithms, actually, to assist in doing such a study. We have a conference paper under review that actually does this although I have to say it’s technically and logistically challenging to do.
Regarding the third point, “Radiation stigma”: I agree, I think the way you are thinking about this is pretty in line with the risk-benefit calculus as far as I understand it. I should have elaborated a bit more, in my post. I was not thinking of doing a screening CT only to screen for kidney stones. I’ve been working with Prof. Pickhardt at UW. One of the things we’ve been researching is the utility of a low-dose “screening CT” in middle age. The screening CT would cover many things including stones. Prof. Pickhardt is working on assembling data to support this idea, mainly focusing on the value of scanning just the abdomen (not the chest). People currently get a coronary calcium score (“CAC”) CT scan for screening their cardiovascular risk. The abdomen also contains biomarkers (like aortic plaque) that also can gauge cardiovascular risk, plus we can look for a lot of other stuff in the abdomen, including kidney stones.
I agree the preventative approach is probably the most promising (identifying patients at-risk using genetics, blood tests, and maybe other factors, not screening CT) .. especially given how safe and cheap potassium citrate is.
See also: The organization for the prevention of intense suffering: https://www.preventsuffering.org/
Wow, great article Dan! It’s good to see such a down-to-earth cause area for the sometimes abstract realm of Suffering-focused EA. . .Is there any data for the number of people suffering from kidney stone pain globally? I saw you give data for the U.S., but I just wonder how prevalent this issue is around the world, and particularly in lower and middle income countries. Thanks!