Summary
An organism’s number of brain cells (e.g., neural processing power) may be a relevant proxy in assessing its likelihood of being sentient and, consequently, its capacity for welfare. In addition, quantitative proxies like brain cell numbers allow for more objective comparisons of moral weight across species, or across developmental stages within the same species.
Isotropic fractionation (IF), a technique recently pioneered for application in insects by Godfrey et al. 2021, allows for insect brain cells to be quickly and reliably counted. IF can be used to determine the number of brain cells in insects, such as the black soldier fly (BSF; Hermetia illucens).
Billions of BSFs are farmed annually across the globe, mainly to be used as animal feed, and the industry is growing. Understanding the likelihood of sentience in BSFs is important due to the massive scale of this new agricultural sector.
My study co-authors and I determined the number of brain cells in adult male and female BSFs as well as L1, L4, and L6 stage larvae. As shown in the paper (preprint here), larvae produced a 9-fold increase in brain cell numbers across larval development. L6 larvae have ~20,000 brain cells. Pupation caused a 16-fold increase in brain cell numbers for adults.
Adult BSFs had an average of ~331,000 brain cells; males and females differed in the number of cells in their brains, due to differences in the optic lobes (peripheral processing regions responsible for the input of visual information). In the central brain, BSF adults had ~42,000 CB cells irrespective of sex.
These data allow for BSF (at multiple developmental stages) to be included in interspecific welfare comparisons that use brain cells as a relevant measure of capacity for welfare.
Caveats
This post assumes sentience in insects is possible, but does not attempt to assess how probable it is based on the data gathered. In addition, it assumes cognitive capacity may be considered a proxy for sentience.
Brain cell counts by themselves provide limited evidence for cognitive capacity, and should be used in conjunction with other behavioral and anatomical data. With insects there is often very little data on these other features (recently reviewed here); brain cell counts may represent an initial foray, then, into understanding a species’ cognitive complexity.
Thus, this research does not assess cognitive sophistication, nor the capacity for welfare in BSF, but may still be of interest to those working to understand BSF sentience.
This post is not meant to examine the utility, or pros/cons, of brain cell numbers as a proxy for cognitive capacity, sentience, or moral weight.
The data reported herein are for total brain cell numbers, which includes non-neuronal cell populations. Data from other Diptera (flies, mosquitoes, etc.) suggest neurons may make up ~90% of all brain cells (Raji & Potter 2021).
Introduction
Between 200 and 300 billion individual black soldier flies (BSFs) are estimated to be farmed annually to be used as animal feed, and the industry is expected to grow (Rowe 2020). The vast majority of farmed BSFs are killed as larvae. Larvae have excellent biomass conversion abilities (Cicková et al. 2015, Lalander et al. 2015), and exchange any waste products they may consume into nutrition for livestock and exotic pets (among other products; Lee et al. 2021, Hopkins et al. 2021, de Souza Vilela et al. 2021).
BSFs belong to the order Diptera, family Stratiomyidae; they are in the same order as the model organism, Drosophila melanogaster (though D. melanogaster belongs to a different family, Drosophilidae). BSFs are native to the Neotropics, but due to globalization have spread across most of the world. In the tropics, BSFs can breed year-round, whereas the number of generations per year in more temperate zones can be influenced by seasonal changes.
Farmed BSF larvae go through six stages (L1-L6) prior to slaughter or pupation. In the industry, pupae are only allowed to eclose (e.g., become adults) if they are destined to be breeders. A review of the literature suggests many welfare concerns exist for farmed BSF larvae and adults (for more on this, view my preprint on BSF welfare, here); these concerns may be targets for intervention by those interested in improving farmed insect lives. However, if BSFs are unlikely to be sentient, the moral significance of these welfare concerns would be negligible. Therefore, it is important to understand the potential capacity for sentience in BSFs to determine if their potential suffering in farmed contexts may merit moral consideration. Additionally, data on the likelihood of BSF sentience may allow us to make comparisons of moral weight across species; these comparisons are important if we wish to most effectively allocate resources to solve welfare concerns for different animal species.
Here, we studied the total number of brain cells (often considered the fundamental unit of neural processing power) in order to better understand BSF sentience. We determined the total number of brain cells in BSF larvae at three different stages (L1, L4, L6), as well as in adult males and females.
Research importance
Given that an estimated 200 to 300 billion black soldier flies are farmed each year (Rowe 2020), they are currently the third most-farmed insect species (by number of individuals reared). Interest in growing the BSF industry is substantial, such that BSFs are likely to become the most farmed insect species within the next decade. By the end of the century, if insects are used in both aquaculture and feedstock, there may be hundreds of trillions of insects farmed each year (Rowe 2020).
If insects are sentient, any welfare concerns in the insects as food and feed industry could represent one of the largest causes of human-associated animal suffering. Our review of current BSF farming practices suggest there are serious welfare concerns present in the industry throughout rearing and during slaughter. In order to determine if these concerns should be addressed, it is critical to know if BSFs are likely to be sentient. Brain cell counts may be an easily-obtained proxy measure for an organism’s cognitive abilities (though it may also be important where in the brain those cells are located). By researching BSF brain cell numbers, and their distribution between peripheral processing lobes (e.g., optic lobes) vs. higher-order regions (e.g., mushroom bodies located in the central brain mass), we may gain a more accurate understanding of their sentience and thus capacity for welfare.
Methods
The full methods can be found in the peer-reviewed publication, in Arthropod Structure and Development, and can also be accessed via the preprint. Using a combination of isotropic fractionation (IF) and immunohistochemistry (IHC), we assessed the number of brain cells in the ventral nerve cords (VNCs) and brains of larvae at stage 1, 4 and 6, and in the optic lobes and central brain regions of male and female adults.
Briefly, isoflurane was used to anesthetize insects prior to fixation. Brains were preserved in Prefer glyoxal fixative for at least 24 hours, then dissected out of the heads (adults) or bodies (larvae); OLs and CBs of adults were weighed separately before IF. VNCs and brains of larvae were also treated separately. For IF treatments, brain tissue was homogenized and brain cell nuclei visualized and counted using SYTOX Green under epiflourescence. IHC (again using SYTOX Green to visualize the brain cell nuclei) was used for the L1 and L4, as brains were too small to reliably dissect out of the larval body in large enough numbers for IF. A 3D image of the brain was taken using a confocal microscope, and every nuclei was counted in the VNCs and brains by at least two separate individuals, using ImageJ, before being averaged.
Essential background on insect brain organization
BSF larval nervous systems (the nuclei of which can be seen in Figure 1C, from the preprint) contain:
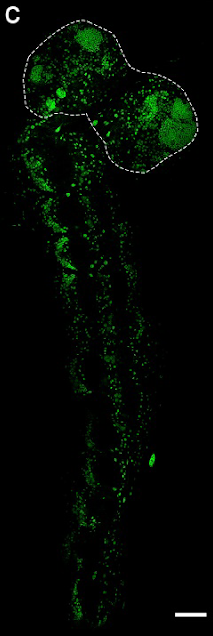
1) a brain consisting of two lobes which house the cells that will divide and become the adult brain (outlined with a dashed line in Figure 1C).
2) a ventral nerve cord (VNC; the equivalent of the vertebrate spinal cord) with 12 segments, responsible for innervating and coordinating each of the twelve body segments (the rest of the green nuclei in Figure 1C, making a ‘tail’ for the two lobes of the brain).
The adult brain has many functionally discrete regions. For this post, two regions should be distinguished:
The optic lobes (OLs), which are a paired region of peripheral processing lobes responsible for the input of visual information from each eye. In BSFs, as is generally true in fast-flying insects, the eyes are quite large and thus the optic lobes are also a very significant part of the brain mass.
The central brain (CB), which houses “everything else” (sensory integration, navigation, memory, learning, chemosensory input, etc.) in the brain.
These regions were handled distinctly in adults, as male BSFs likely use visual cues for locating and mating with females, suggesting there may be behavioral reasons for sexual dimorphism in the number of OL cells (Oonincx et al. 2016, Tomberlin & Sheppard 2002, Tingle 1975, Liu et al. 2020, Zhang et al. 2010, Macavei et al. 2020, Klüber et al. 2020, Heussler et al. 2018, Holmes 2010, Nakamura et al. 2016, Schneider 2020).
Summary of major findings
Data on brain mass, allometric scaling, and more can be found in the full preprint (and all the raw data can be accessed here, on Dryad, for any individuals that may wish to do more analyses). Here, I review the results most important for those looking to use brain cell counts as a proxy for cognitive ability, or as a feature potentially indicative of the capacity for conscious experience.
L1 larvae had ~2,300 brain cells, which increased 9-fold over development to a total of ~20,000 brain cells for L6 larvae.[1] Adults had an average of ~331,000 cells in their brain, representing a 16-fold increase during pupation.
Males and females had statistically different numbers of cells, due to differences in the OLs (male and female CBs had similar cell numbers).
Males had ~322,000 cells in their OLs while females had 258,000 cells. Adults had an average of ~42,000 cells in their CB regardless of sex.
Males may have increased OL cell numbers due to the importance of visual information to their mating behaviors (Oonincx et al. 2016, Tomberlin & Sheppard 2002, Tingle 1975, Liu et al. 2020, Zhang et al. 2010, Macavei et al. 2020, Klüber et al. 2020, Heussler et al. 2018, Holmes 2010, Nakamura et al. 2016, Schneider 2020). Male honey bees also have increased numbers of OL cells, compared to female workers, likely for this reason (Streinzer et al. 2013, Witthöft 1967).
If brain cell numbers are a good proxy for cognitive ability, it would be reasonable to assume D. melanogaster and BSFs may have similar cognitive capacities (though future research on the distribution of brain cells, as well as synapse numbers, across functionally discrete regions, would be useful, as well as data on allometric scaling of brain cell numbers across numerous Diptera).
L1 D. melanogaster larvae have ~2,000 cells; L3 (final D. melanogaster larval stage) have ~8-10,000 cells (Scott et al. 2001, Nassif et al. 2002, Avalos et al. 2019).
L1 BSF have~2,300 cells; L4 BSF have ~13,000 cells; L6 BSF have ~20,000 cells.
Adult D. melanogaster have ~93,000 − 133,000 cells (the highest estimate is 199,000 to 208,000 cells; Scheffer et al. 2020, Godfrey et al. 2021, Raji & Potter, 2021, Mu et al. 2022).
Adult BSFs have an average of ~331,000 cells in their brain.
Notably, cell numbers in the CB are likely to be a better indicator of cognitive ability than total cell numbers, as the OL cells are primarily centers for the input of visual information only.
BSFs had ~42,000 cells (did not differ by sex); estimates for D. melanogaster vary widely from ~43,000 to 101,000 cells—until this extreme variation is resolved, an interspecific comparison of D. melanogaster and BSFs based on CB cell numbers alone is challenging. [However, my personal expectation, based on other data in the papers providing these estimates, is that CB numbers in D. melanogaster are much closer to 43,000 (Mu et al. 2022) than 101,000 (Raji & Potter 2021) cells].
Future Directions
We do not have brain cell numbers for many other farmed invertebrate species, including: mealworms, lesser mealworms, silkworms, waxworms, superworms, lac bugs, crickets (Gryllus assimilis, Gryllodes sigillatus, and Acheta domesticus), grasshoppers (Sphenarium sp.), or prawns (farmed: Litopeneaus vannamei and Penaues monodon; wild-caught: Acetes japonicus). Data on these species may provide some evidence for cognitive ability and, indirectly, sentience (although cross-species comparisons should be made carefully, and with principles of allometric scaling in mind). Researchers that have an interest in applying isotropic fractionation to other invertebrate animals may wish to reach out to me (meghan@rethinkpriorities.org). As with all scientific methodologies, many specific details about optimizing the protocols do not appear in the published papers. I can advise about what I learned in the process of collecting data for this publication, or help troubleshoot challenges faced while employing these methods.
In addition, better resolution of the number of cells in the CB of D. melanogaster (estimates of the total number vary from ~43,000 to 101,000 cells, e.g. Mu et al. 2022, Raji & Potter 2021) would be useful for modest comparisons of the cognitive capabilities of BSFs to D. melanogaster (assuming CB cell numbers roughly relate to cognitive capabilities across Dipterans). Importantly, there are notable exceptions to the rule that brain cell numbers correlate with cognitive ability (Schukraft 2019). For this reason, a better understanding of the distribution of cells across regions within the CB, as well as cell types and connectivity, would also be helpful in making comparisons between species.
Especially interesting would be to assess how brain cell numbers change across development in holometabolous (metamorphosing) versus hemimetabolous (non-metamorphosing) insect species, as different developmental rules may regulate the division of neural precursor cells in species with such vastly different developmental paradigms. Systematic collection of this data (particularly if collected across multiple taxa) could lead to more confident generalizations of brain cell numbers for many less-commonly-studied insect species at different life stages. This data would be relevant to those interested in the sentience of both immature and mature insects, especially in wild insect welfare situations, where data on brain cell numbers will not be available for the vast majority of species.
A combination of IF and IHC may be useful in analyzing the number of cells in different regions of animal brains. For example, differences in OL numbers caused a difference in the total number of brain cells in adult male and female BSFs, however the OLs may not be the most relevant brain region when considering their respective likelihood of sentience (the OLs generally only handle the input of visual information from each eye to the central brain region). By contrast, the number of cells in the mushroom bodies (centers of sensory integration, learning, and memory) may be more relevant in determining/comparing potential cognitive sophistication. Brain-region-specific cell, or synapse, counts may prove to be more reliable metrics than total brain cell numbers. Ideally, future research would assess cognitive ability directly in relation to cell numbers in many insect taxa, to assess if this proxy is truly a good measure for cognitive function across species. This would supplement a fundamental limitation of this dataset—namely, that we are unsure how good total cell numbers may be as a proxy for cognitive capacity.
Future studies on BSFs specifically are also needed to better assess their capacity for sentience; as BSF is farmed in large numbers, it is critically important for this specific species to be studied in-depth for features relevant to sentience. Rethink Priorities has already written many useful resources about sentience-relevant features in invertebrates, and studies addressing any of these behavioral or anatomical features in BSF larvae and adults will be important for determining their degree of cognitive sophistication and capacity for sentience.
Conclusion
Hundreds of billions of BSFs are farmed annually across the globe to be used, mainly, as livestock feed, and the industry is growing. Given that many welfare concerns have been identified for farmed BSFs, if insects are sentient, this industry will represent one of the fastest-growing causes of human-induced animal suffering. Understanding the potential for sentience in BSFs will be crucial for assessing the impact of the industry on animal welfare. An organism’s number of brain cells may be a relevant proxy in assessing its likelihood of being sentient (and/or its cognitive abilities) and, consequently, its capacity for welfare. In addition, quantitative proxies like brain cell numbers allow for more objective comparisons of moral weight across species, or across developmental stages within the same species. In this study, we determined the number of brain cells in adults (used as breeders; ~331,000 cells) and larvae across development (L1: 2,300 cells; L6: 20,000 cells). We compared these data to brain cell numbers in D. melanogaster, a species about which more data on behavior and cognitive abilities is available. We concluded that, if brain cell numbers are a good proxy for cognitive ability, then it would be reasonable to assume D. melanogaster and BSFs may have similar cognitive capacities (though future empirical research on brain cell numbers as a cognitive proxy will be necessary). Ultimately, further research on the behaviors and neurobiology of BSFs will be necessary to better understand their capacity for sentience.
Acknowledgments
This post summarizes the results of a prior research collaboration between Rethink Priorities and external academic collaborators. R. Keating Godfrey, Emily Sterner, and Edward Waddell all assisted in the data collection process; they are all co-authors on the resulting peer-reviewed publication. R. Keating Godfrey created panel 1C for the preprint, which did not end up in the final publication and is thus reproduced in this post; Patty Jansma and the University of Arizona Imaging Core Optical facility provided support for this photo. Daniela R. Waldhorn and Jason Schukraft both contributed to conversations about the utility of brain cell counts; Daniela also contributed to the review and editing of this post.
This post was written solely by Meghan Barrett, and is not necessarily representative of the opinions or expertise of the other study co-authors, who have not reviewed it. Barrett is currently an NSF postdoctoral fellow: any opinions, findings, conclusions, or recommendations expressed in this manuscript are the author’s, and do not necessarily reflect the views of the NSF.
If you are interested in Rethink Priorities’ work, please visit our research database and subscribe to our newsletter.
References
Avalos CB, Maier GL, Bruggmann R, Sprecher SG. 2019. Single cell transcriptome atlas of the Drosophila larval brain. eLife, 8: e50354.
Bessa, L.W., Pieterse, E., Marais, J., Dhanani, K., and Hoffman, L.C., 2021. Food safety of consuming black soldier fly (Hermetia illucens) larvae: Microbial, heavy metal, and cross-reactive allergen risks. Foods 10: 1934.
Cappellozza, S., Leonardi, M.G., Savoldelli, S., Carminati, D., Rizzolo, A., Cortellino, G., Terova, G… Tertamanti, G., 2022. A first attempt to produce proteins from insects by means of a circular economy. Animals 9: 278.
Chia, S.Y., Tanga, C.M., Khamis, F.M., Mohamed, S.A., Salifu, D., Sevgan, S., Fiaboe, K.K.M, Niassy, S., van Loop, J.J.A., Dicke, M. and Ekesi, S., 2018. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PloS ONE 13: e0206097.
Cicková, H., Newton, G.L., Lacy, R.C., and Kozánek, M., 2015. The use of fly larvae for organic waste treatment. Waste Management 35: 68-80.
de Souza Vilela, J., Andronicos, N.M., Kolakshyapati, M., Hilliar, M., Sibanda, T.Z., Andrew, N.R., Swick, R.A., Wilkinson, S., and Ruhnke, I., 2021. Black soldier fly larvae in broiler diets improve broiler performance and modulate the immune system. Animal Nutrition 7: 695 – 706.
European Food Safety Administration, 2015. Risk profile related to production and consumption of insects as food and feed. EFSA Journal 13: 4257.
Food and Agricultural Organization of the United Nations. 2021. Looking at edible insects from a food safety perspective. Challenges and opportunities for the sector. Rome. Available at: https://doi.org/10.4060/cb4094en
Godfrey RK, Swartzlander M, Gronenberg W. 2021. Allometric analysis of brain cell number in Hymenoptera suggests ant brains diverge from general trends. Proceedings of the Royal Society B 288: 20210199.
Heussler CD, Walter A, Oberkofler H, Insam H, Arthofer W, Schlick-Steiner BC, Steiner FM. 2018. Influence of three artificial light sources on oviposition and half-life of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae): Improving small-scale indoor rearing. PLoS One, 13(5): e0197896.
Holmes L. 2010. Role of abiotic factors on the development and life history of the Black Soldier Fly, Hermetia illucens (L.) (Diptera: Stratiomyidae). [PhD Dissertation]. University of Windsor.
Hopkins, I., Newman, L.P., Gill, H., and Danaher, J., 2021. The influence of food waste rearing substrates on black soldier fly larvae protein composition: A systematic review. Insects 12: 608.
Klüber P, Bakonyi D, Zorn H, Rühl M. 2020. Does light color temperature influence aspects of oviposition by the black soldier fly (Diptera: Stratiomyidae)? Journal of Economic Entomology, 113: 2549-2552.
Lalander, C., Fidjeland, J., Diener, S., Eriksson, S., and Vinnerås, B., 2015. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agronomy for Sustainable Development 35: 261–271.
Liu Z, Najar-Rodriguez AJ, Minor MA, Hedderley DI, Morel PCH. 2020. Mating success of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae) under four artificial light sources. Journal of Photochemistry & Photobiology, B, 205: 111815.
Lee, K.S., Yun, E.Y., and Goo, T.W., 2021. Optimization of feed components to improve Hermetia illucens growth and development of oil extractor to produce biodiesel. Animals 11: 2573.
Macavei LI, Benassi G, Stoian V, Maistrello L. 2020. Optimization of Hermetia illucens (L.) egg laying under different nutrition and light conditions. PLoS ONE, 15: e0232144.
Moretta, A., Salvia, R., Scieuzo, C., di Somma, A., Vogel, H., Pucci, P., Sgambato, A., Wolff, M., and Falabella, P., 2020. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Scientific Reports 10: 16875.
Mu S, Yu S, Turner NL, McKellar CE, Dorkenwald S, Collman F… Seung HS. 2022. 3D reconstruction of cell nuclei in a full Drosophila brain. Preprint available at: https://www.biorxiv.org/content/10.1101/2021.11.04.467197v1
Nakamura S, Ichiki RT, Shimoda M, Morioka S. 2016. Small-scale rearing of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), in the laboratory: low-cost and year-round rearing. Applied Entomological Zoology, 51: 161-166.
Nassif C, Noveen A, Hartenstein V. 2002. Early development of the Drosophila brain: III. The pattern of neuropile founder tracts during the larval period. Journal of Comparative Neurology, 455: 417-434.
Oonincx DGAB, Volk N, Diehl JJE, van Loon JJA, Belušíč G. 2016. Photoreceptor spectral sensitivity of the compound eyes of black soldier fly (Hermetia illucens) informing the design of LED-based illumination to enhance indoor reproduction. Journal of Insect Physiology, 95: 133-139.
Raji JI, Potter CJ. 2021. The number of neurons in Drosophila and mosquito brains. PLoS ONE 16: e0250381.
Rowe A. 2020. Insects raised for food and feed—global scale, practices, and policies. Effective Altruism. Retrieved from: https://forum.effectivealtruism.org/posts/ruFmR5oBgqLgTcp2b/insects-raised-for-food-and-feed-global-scale-practices-and#Black_soldier_flies1. Last accessed on 1/24/2022.
Scheffer LK, Xu SC, Januszewski M, Lu Z, Takemura S, Hayworth KJ… Maitlin-Shepard J. 2020. A connectome and analysis of the adult Drosophila central brain. eLife, 9: e57443.
Schneider JC. 2020. Effects of light intensity on mating of the black soldier fly (Hermetia illucens, Diptera: Stratiomyidae). Journal of Insects as Food and Feed, 6: 111-119.
Schukraft J. 2019. Features relevant to invertebrate sentience, part 1. Rethink Priorities. Retrieved from: https://rethinkpriorities.org/publications/features-relevant-to-invertebrate-sentience-part-1 Last accessed on 7/14/2022.
Scott K, Brady R, Craychik A, Morozov P, Rzhetsky A, Zuker C, Axel R. 2001. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell, 104: 661-673.
Streinzer M, Brockmann A, Nagaraja N, Spaethe J. 2013. Sex and caste-specific variation in compound eye morphology of five honey bee species. PLOS One, 8: e57702.
Tingle FC, Mitchell ER, Copeland WW. 1975. The soldier fly, Hermetia illucens, in poultry houses in North Central Florida. Journal of the Georgia Entomological Society, 10: 179-183.
Tomberlin JK, Sheppard DC. 2002. Factors influencing mating and oviposition of Black Soldier Flies (Diptera: Stratiomyidae) in a colony. Journal of Entomological Science, 37: 345-352.
Vogel, H., Müller, A., Heckel, D.G., Gutzeit, H., and Vilcinskas, A., 2018. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Developmental and Comparative Immunology 78: 141-148.
Witthöft W. (1967). Absolute Anzahl und Verteilung der Zellen im Hirn der Honigbiene. Z Morph Tiere 61:160–184.
Zhang J, Huang L, He J, Tomberlin JK, Li J, Lei C, Sun M, Liu Z, Yu Z. 2010. An artificial light source influences mating and oviposition of black soldier flies, Hermetia illucens. Journal of Insect Science, 10: Article 202.
- ^
Larval development (of any holometabolous insect) is highly dependent on diet and other abiotic conditions, such as temperature. In BSFs, development times can be as short as 14 days with good nutrition and high rearing temperatures, or much longer diunder fferent nutritional/abiotic programs. For example, a vegetable- and fruit- substrate diet (Cappellozza et al. 2019) increased development times in the lab to 45 days, while rearing at cool temperatures increased development time to an excess of 60 days (Chia et al. 2018). Longer developmental periods may be reported for even less hospitable diets/conditions.
This is really fantastic work! I really love seeing this kind of basic science being done in animal welfare, since it’s so critical to being able to discuss and evaluate interventions.
It’s worth mentioning that there’s a company called Insectta trying to use black soldier fly larvae (which might differ quite a lot in terms of neuron counts, neural structure, and probability of sentience, than their adults) to produce semiconductors.