Treating Cluster Headaches Using N,N-DMT and Other Tryptamines
To extend this recent EA Forum Post, I wanted to share the results of Qualia Research Institute’s research into using tryptamines to abort and prevent cluster headaches. While the quotes and statistics contained here can provide some notion of the pain experienced by cluster headache sufferers, I think it is truly unimaginable. This report contains specific interventions to be pursued in both a philanthropic and for-profit business capacity. While for-profit options are beyond our scope, those interested in supporting philanthropic interventions should consider donating to ClusterBusters (the most important nonprofit dedicated to researching treatments for cluster headaches), or QRI (which does foundational research on ways to reduce intense suffering).
Mission: Instantly and safely abort cluster headaches and treat migraines, the #2 and #10 (respectively) most painful medical conditions according to NHS. Emphasis is placed on chronic cluster headaches, which account for as much as 80% of all clusters and currently lack an effective treatment option.
I. Problem:
“Even child birth is 1/10th the pain of a cluster headache, seriously this name needs to change… call it ultra super migraine.” (source)
A back of the envelope calculation indicates there are roughly 14 thousand people enduring a cluster headache right now.[1]
14.2% of US adults 18 or older reported having migraine or severe headache in the previous 3 months in the 2012 NHIS. The overall age-adjusted 3-month prevalence of migraine in females was 19.1% and in males 9.0%, but varied substantially depending on age. (source)
Current treatments are either ineffective, costly, unsafe, or some combination of the three. The most effective treatments available for cluster headaches include oxygen, which requires the patient to carry an oxygen tank with them at all times, and triptans, which can be used a maximum of three times daily (an issue for chronic sufferers especially) and have side effects from pain to heart attack and stroke. The most effective treatments for migraines include triptans and opioids (which have high addiction potential). Emgality, a more promising treatment for episodic cluster headaches, has recently entered the market, but its long-term risk profile and efficacy have not yet been established.
Bob Wold founded “Cluster Busters” in 2002 with the explicit purpose of trying to get psychedelics to be prescription medication (see his lecture Treating Cluster Headaches with Psychedelics). He tried over 75 different prescription medications and was at the end of the rope when he found psychedelics could be helpful:
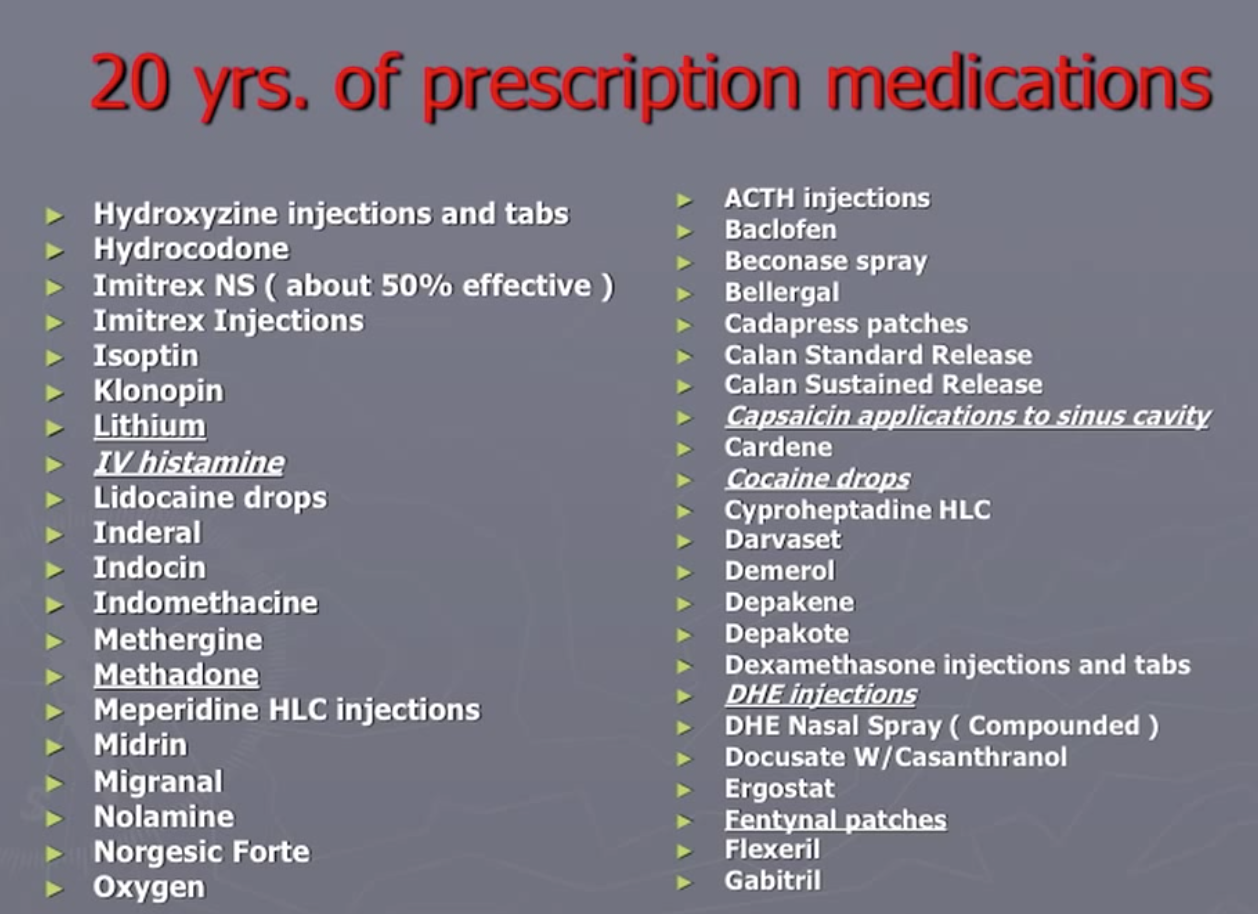
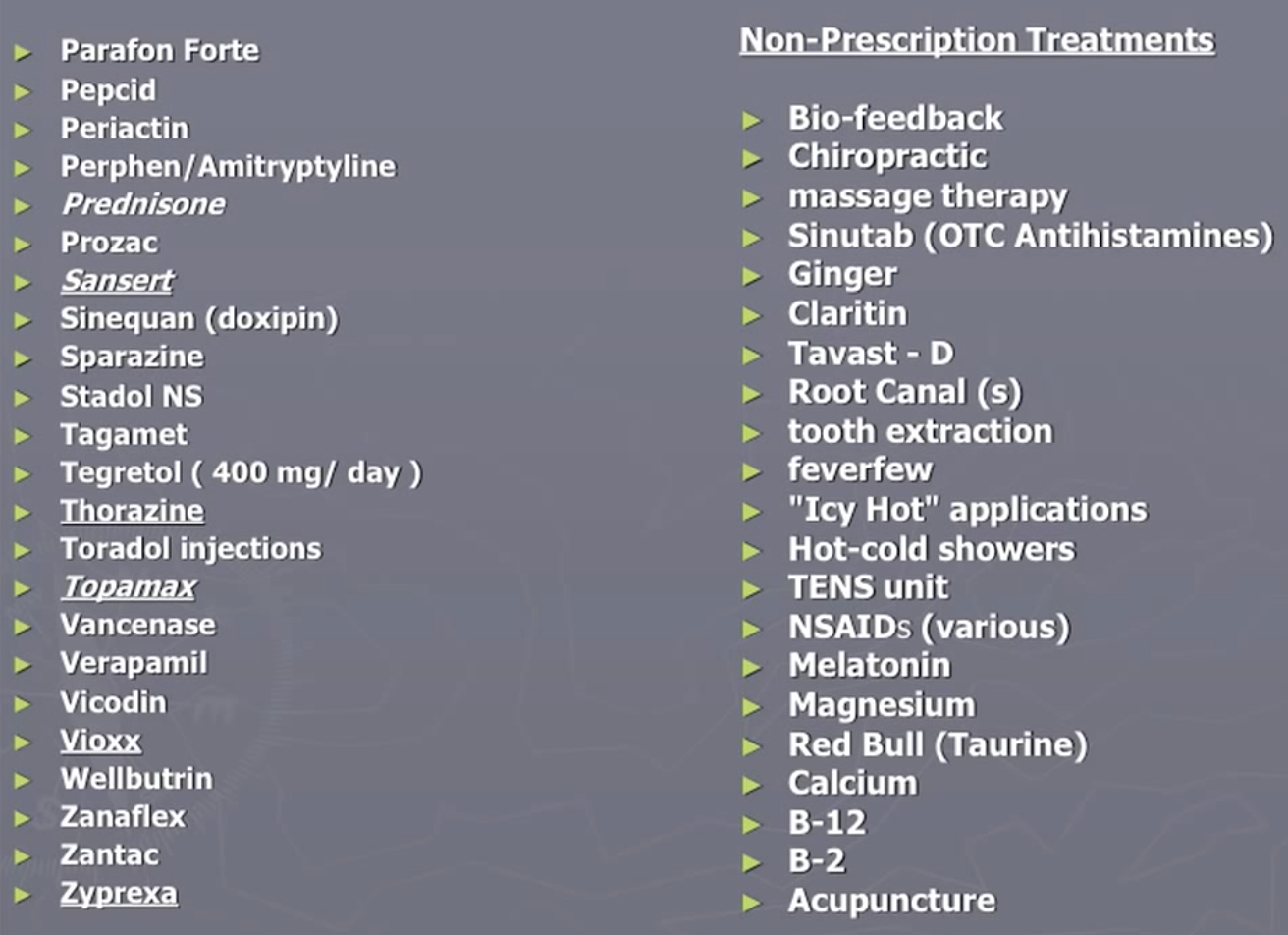
As noted in this Qualia Computing article, the survey surfaced that about 83% of all cluster headaches are experienced by 20% of the sufferers, most of which are classified as ‘chronic’. No existing medication has been approved for use to treat chronic cluster headaches. Vaporizing DMT could be the first such treatment, offering instant relief for cluster headaches as often as they arise in a (potentially large) percentage of sufferers.
II. Solution:
“One of the most incredible experiences of my life was when I first aborted a CH [cluster headache] with DMT. That feeling of going from a place of excruciating pain…and feeling the pain fizzle away and die in a matter of seconds” (source)
It is known by a majority of cluster headache sufferers that psychedelics can be highly effective treatments. Due to the legal status of psychedelics, no randomized controlled trial (RCT) has been conducted, but analysis has been done on online forum responses and anonymous surveys, and interviews have been recorded. Evidence from these reports points to a number of important factors: tryptamines (the class of psychedelics which includes compounds like N,N-DMT and psilocybin, the active chemical in ‘magic mushrooms’) seem especially effective, sub-psychoactive and non-psychedelic doses can be therapeutic, and psychedelics can also decrease the frequency of headaches on long time horizons. While smoking/vaporizing is the fastest method of administration available, information from a private correspondence suggests that the FDA may be averse to approving inhalants. Intramuscular administration, utilizing Rick Strassman’s protocol, could be an alternative that would achieve rapid relief without the use of an inhalation device[2]. Since the pain being experienced is so severe, having a faster method of administration is critical.
From the relevant academic literature:
“The indoleamine hallucinogens, psilocybin, lysergic acid diethylamide, and lysergic acid amide, were comparable to or more efficacious than most conventional medications. These agents were also perceived to shorten/abort a cluster period and bring chronic cluster headache into remission more so than conventional medications.” (source)
“Also, for DMT, it was suggested that singular or infrequent dosage could have potential long-term beneficial effects on headache disorders: ‘Even a single dose, or perhaps a couple, can be a lifelong benefit.’” (source)
“Of interest, an open-label study found that similar compounds (2-bromo-LSD) without psychedelic effect were promising for this purpose” (source)
From online surveys:
A survey of members of online cluster headache forums revealed that 68% of respondents who used tryptamines had a 4 or 5 out of 5 relief. 5 indicates “completely eliminated the cluster headaches”.
This survey again suggests that the main barrier to use is lack of access and hallucinogenic effects. As we found in an interview with an anonymous sufferer (see below), hallucinogenic effects may be avoidable.
Difficulty getting. 0 – Extremely easy to acquire, 5 – Nearly impossible to acquire
Legal risk. 0 – Not concerned at all, 5 – Extremely concerned
Side effects. 0 – Not concerned at all, 5 – Extremely concerned
From interviews with cluster headache sufferers who have tried N,N-DMT:
“If I’m starting to experience a CH and I vaporize as little as 3mgs [of DMT, sub psychoactive] I feel the pain subside almost completely within 3 to 5 seconds, and it doesn’t come back for a few days.” – (full document; also: another interview with an anonymous cluster headache sufferer)
III. Philanthropic Opportunities:
Due to the Schedule I status of psychedelics in the United States, pursuing this intervention in the US will not be feasible for a number of years (see Section IV for more information on pursuing FDA approval for DMT for cluster headaches/migraines).
A possible solution would be to create an online education campaign publicizing the results of this report to cluster headache sufferers, designated as ‘information-only’, and pursuing the use of psychedelics to treat cluster headaches and migraines in countries where tryptamines are legal, including Brazil, Jamaica and the Netherlands. In addition, given the gravity of the disorder, it could be cost-effective to fly patients to such countries for months at a time.
While we believe that traditional metrics such as the QALY do not accurately capture the suffering caused by a cluster headache (see upcoming post on the true pain/pleasure scale), a rough QALY calculation would be as follows (focusing on chronic cluster headache sufferers rather than average, since they compromise up to 83% of total headaches[3]):
Facebook AD campaign:
An estimated 370,000 Americans suffer from cluster headaches, 68% of whom are on Facebook[4] (=251,000). About 15% of these suffer from chronic cluster headaches (=37,740). According to Sprout Social, the average estimated cost per click of an ad campaign is $1.72. Assuming 1⁄10 who click are cluster headache sufferers, to reach all chronic sufferers would take (ballpark) $650,000.
Assuming about 30% of those who view the ad will pursue the treatment (rough estimate-those who put 2 or less on survey results for questions of legality, difficulty to acquire etc.) and that in 68% of cases it cured or nearly cured their clusters (based on survey results), then the resulting increase in QALYs would be (37,740 people * 0.3 * 0.68) * [0.760 (QALY coefficient) * 1 QALY – ( −0.429 (QALY coefficient)* (0.47QALY)) [5]] = $650,000/7, 404QALY = $87.70 per QALY.
These ads could also be targeted to users in countries where psilocybin and DMTare legal for use recreationally, increasing conversion rate. Further targeting could be done on Facebook groups (and other social media groups) which are associated with cluster headache treatment.
IV. For-Profit Opportunities:
The recent emergence of psychedelics in for-profit business settings also affords the opportunity for entrepreneurs to seek legal rescheduling of N,N-DMT in the US for the purpose of treating cluster headaches and/or migraines. Below is an outline of the process of navigating the FDA IND process, which could result in a change in legal status:
Market:
‘Orphan disease’ status:
There are two main classifications of cluster headaches, chronic and episodic. Episodic cluster headaches are characterized by periods of headaches (up to 8 times per day) of a week to a full year, which remit for periods from a month up to a year. Chronic cluster headaches, on the other hand, either last for longer than a year or have remittance periods of less than a month[6]. A meta-analysis from the NIH estimates that cluster headaches affect 124⁄100,000 in the U.S., meaning an estimated 370,000 people suffer from cluster headaches a year[7]. Of these, about 15%, or 60,000, suffer from chronic cluster headaches.
The FDA grants ‘orphan disease’ status to diseases which affect fewer than 200,000 persons in the U.S per year, and offers incentives to those pursuing treatments through the FDA’s IND process for such diseases, such as longer periods of exclusivity (monopoly on drug manufacture and sale) for the treatment after approval.
The global market for migraine drugs (which encompasses cluster headache drugs) in 2017 was $1.7 billion.
Healthcare and lost productivity costs associated with migraine are estimated to be as high as $36 billion annually in the U.S. Current estimates of cluster headaches’ annual cost in the U.S. is ~$3.5 billion.
Share of market
5 years after launch (with FDA approval, with a 5-year monopoly) – serve 20% of chronic migraine sufferers (800,000), serve 20% of cluster headache sufferers (40,000)
Platform’s average annual revenue per patient (migraines): $452/patient/year
DMT Vape Pen – $20
1g of DMT is ~$100, approximately 50 doses (although an anecdote indicate 3mg may be sufficient). Compare to triptans, at ~$115 per 9 doses. Assuming 20% markup:
Chronic migraines at 20mg doses: $120/g*0.02g/dose*15 doses/month *12 months = $432/patient/year.
Platform’s average annual revenue per patient (cluster headache): $344/patient/year-low estimate, $6932/patient/year – high estimate
DMT Vape Pen – $20
There is significant variance in frequency of cluster headaches: estimates range from [$120/g*0.02g/dose*30 doses/month *3 months = $324, $120/g*0.02g/dose*120 doses/month*12 months = $6912/patient/year].
Annual revenue, 5 years after launch: $13.6M [low cluster headache estimate] – $344M [high cluster headache estimate]
Annual revenue, 5 years after launch (migraines): $344M
The 5-year (or more, if ‘orphan disease’ status is gained) monopoly provided by the FDA would allow for further R&D, and as-yet undetermined projects. Some promising directions:
Depression treatment ($13.7B market)
DMT may be beneficial for tobacco cessation ($7.0B market).
Ayahuasca shows significant evidence for efficacy in treating addiction.
There is anecdotal evidence that vaporized DMT is also effective in this space.
Why now?
FDA on track to approve MDMA therapy in 2021, psilocybin therapy in 2022
FDA approval will catalyze a large increase in demand for psychedelic services
There is sufficient evidence to attempt bringing DMT for headaches through the FDA process as it becomes increasingly open to psychedelic interventions
Reasons to start before FDA approval of MDMA and psilocybin:
A “psychedelic renaissance” is underway: funding for psychedelic research has skyrocketed, and multiple psychedelic decriminalization initiatives (1, 2) have recently passed. Riding the current wave of activist and public support is advantageous to our efforts.
More time to build relationship with the FDA (important for seeking DMT clearance)
More time to build relationships with organizations currently seeking FDA approval for therapeutic uses of psychedelics (MAPS & Compass Pathways)
Challenges:
Regulatory:
Taking on the FDA IND process can be challenging and high risk from an investment standpoint. The average cost of successfully completing Phase 1-3 trials (after which the drug can be rescheduled and approved for medical use) is $100m, requires about 9-11 years and has a 6.7% success rate (private correspondence).
The Multidisciplinary Association for Psychedelic Studies (MAPS) has recently raised $26.7M for Phase 3 MDMA trials alone[8]. Total, MAPS has spent in the ballpark of $30M. If Phase 3 trials demonstrate statistically-significant results, MDMA could be selectively rescheduled for use in therapeutic settings, but would require subsequent Phase 4 trials.
The FDA is risk-averse and has incurred backlash from their last notable rescheduling of fentanyl in 1985[9]. Convincing the FDA to pursue rescheduling for treatment of a relatively rare disease with other available medications will likely be difficult.
The success or failure of MAPS in receiving approval for MDMA will be crucial for defining the regulatory landscape for other psychedelics. Should they fail, bringing another similar substance through the process may prove much more difficult.
Competition:
As discussed in Section I, most available migraine and cluster headache drugs are ineffective, expensive, and/or have heavy risk profiles. Emgality, a new migraine drug approved last month, has received FDA ‘breakthrough therapy’ status for its ability to decrease the frequency of episodic cluster headaches and has shown promise as a palliative for migraines as well[10]. Emgality has not been approved for use in treating chronic cluster headaches, however, and does not achieve the same rapidity of administration as the DMT vape pen (see Section III). Thus, our solution is still critical for relieving symptoms instantly, and maintains the advantage of being eligible to treat chronic cluster headaches, an ‘orphan disease’.
Business model:
We would design studies to fulfill the three-step FDA drug review process:
Phase 1 studies (typically involve 20 to 80 people).
Phase 2 studies (typically involve a few dozen to about 300 people).
Phase 3 studies (typically involve several hundred to about 3,000 people).
Use of Funds
Expenses for research and operations staff
Technicians
Analysis consultants
Researchers with clinical experience
Legal counsel (paperwork)
Phase 1 FDA trial (our connections to expertise in the field would reduce the cost compared to average Phase 1 trials)
Data on Cost of Trials
The following information is from the MDMA/PTSD Trials led by MAPS. However, the treatment for PTSD involves: multiple therapy sessions and an MDMA-trained psychotherapist. Therapy sessions also last 6-8 hours. Presumably, some of these costs would not apply to a DMT/CH trial, so we expect trials for DMT/CH to be cheaper than the MDMA/PTSD Trials.
However, cluster headaches are not well suited to the therapeutic environment that is used to treat mental health conditions (they arise unpredictably, and require instant relief). This means there are likely significant cost-saving opportunities in the experimental design protocol.
Summary of costs for MAPS IND Process:
[1] Assume a world population of 7.7 billion people, and 53 out of 100,000 yearly prevalence suffering from this. Going by public health records, we see that the average number of cluster headache attacks that a sufferer experiences is about 30 a year (with a huge variance, where some people get only about 5 a year and some get them multiple times a day). Attacks last on average 1 hour (but range from 20 minutes to 3 hours). Hence, the number of people currently experiencing a CH is: 0.00053*7,700,000,000*(30/(24*365)) = 13,976.03 ~= 14 thousand
[2] Perspectives on DMT Research
[3] According to survey
[4] https://www.facebook.com/business/help/1461718327429941
[5] For chronic sufferers, an average of between 1-8 CH per day, 1-4 hours per CH, for ~0.47 years/year having CH
[7] https://www.ncbi.nlm.nih.gov/pubmed/18422717
[8] https://maps.org/research/mdma/ptsd/phase3/timeline
[9] https://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf
- Quantifying the Global Burden of Extreme Pain from Cluster Headaches by (1 Nov 2024 20:35 UTC; 211 points)
- “Most painful condition known to mankind”: A retrospective of the first-ever international research symposium on cluster headache by (18 May 2025 11:23 UTC; 163 points)
- Emerging evidence on treating cluster headaches with DMT by (18 Oct 2025 14:32 UTC; 123 points)
- Qualia Research Institute: History & 2021 Strategy by (25 Jan 2021 21:38 UTC; 81 points)
- The Quest for a Stone-Free World: Chanca Piedra (Phyllanthus niruri) as an Acute and Prophylactic Treatment for Kidney Stones and Their Associated Extreme Negative Valence by (18 Feb 2025 0:08 UTC; 76 points)
- What are the key ongoing debates in EA? by (8 Mar 2020 16:12 UTC; 74 points)
- What are the key ongoing debates in EA? by (8 Mar 2020 16:12 UTC; 74 points)
- OPIS initiative on access to psilocybin for cluster headaches by (10 Sep 2020 8:22 UTC; 62 points)
- How much should we value averting a Day Lived in Extreme Suffering (DLES)? by (26 Jul 2025 21:02 UTC; 60 points)
- Qualia Research Institute: History & 2021 Strategy by (LessWrong; 25 Jan 2021 21:46 UTC; 39 points)
- 's comment on Logarithmic Scales of Pleasure and Pain: Rating, Ranking, and Comparing Peak Experiences Suggest the Existence of Long Tails for Bliss and Suffering by (11 Jan 2022 20:25 UTC; 29 points)
- 's comment on The Future Fund’s Project Ideas Competition by (8 Mar 2022 6:09 UTC; 8 points)
- 's comment on Cause X Guide by (1 Sep 2019 20:29 UTC; 8 points)
Leaving this here: “Testimonial of a chronic cluster headache patient after using DMT to abort attacks”. @jonleighton of the Organisation for the Prevention of Intense Suffering interviews John Fletcher, chronic cluster headache patient.
JF: I’m 65 years old now and they [cluster headaches] started for me in late September 1973, so about 51 years I’ve had cluster headaches. […] I came out of a seven-year remission and as soon as I did, I haven’t had a break since, and that was 6 months ago. I’ve been getting at least 10 attacks a day, every day.
JL: Can you tell us a little bit more about the other medical conditions that you’re suffering from at the moment, just to give a bit of context?
JF: Besides stage five COPD [chronic obstructive pulmonary disease], I have adenocarcinoma lung cancer. I have achalasia disease, which my esophagus swells up and ultimately causes me to aspirate food and causes bacterial pneumonia. And I’ve got multiple abdominal hernias from surgery. I had 20 major surgeries. I broke my back at the beginning of the year that was caused by severe osteoporosis from steroids, from my COPD. And I’ve broken my ribs 30 or 40 times in the last couple of years from severe osteoporosis.
JL: How would you compare the suffering due to cluster headaches compared to everything else that you’re experiencing?
JF: I wouldn’t trade anything for cluster headaches. None of it. Cluster headaches is the worst pain I’ve ever had in my life. I’ve never felt anything worse. And, I mean, including being terminally ill and breaking and just so many other really severe painful conditions, but cluster headaches is in a category by itself. I’ve never felt anything like it. It’s such severe pain, it’s literally violent screaming pain and, to put it bluntly, it feels like you’re being murdered. It’s unbelievable pain. And you expect to see blood. Telling people about my first attack, I thought I was shot in the head. I thought somebody shot me and I was dying. Every attack pretty much feels like you’re dying. It’s just horrible, horrible thing. So no, I wouldn’t trade any of it for cluster headaches.
JL: And what medications have you tried? And have any of them worked for you over the years?
…
As explained in the review of Log Scales, cluster headaches are some of the most painful experiences people can have in life. If a $5 DMT Vape Pen produced at scale is all it takes to fully take care of the problem for people sufferers, this stands to be an Effective Altruist bargain.
In the future, I would love to see more analysis of this sort. Namely, analysis that look at particular highly painful conditions (the “pain points of humanity”, as it were), and identify tractable, cost-effective solutions to them. Given the work in this area so far, I expect this to generate dozens of interventions that, in aggregate, might take care of perhaps even the majority of dolors experienced by people.
Exciting to see the update in that post that some studies of LSD/tryptamines and cluster headaches are underway! Tragic as always that medical approval is so slow, and experimentation in all aspects of society is generally so under-supplied.
It seems like psilocybin is thought to have similar effects? Since that has already been legalized for medical use in Oregon, I could imagine setting up a small business or medical practice there to promote psilocybin as a cluster-headache cure (if it actually works, of course). I imagine that the obstacle here is that psilocybin is only approved for certain medical uses—PTSD, end-of-life care—and cannot be prescribed off-label for cluster headaches. But maybe it’s a doable plan, or perhaps Oregon legislators could be convinced to tweak the regulations? (The fact that Oregon has legalized psilocybin might also be a good reason to do a cheaper and more concentrated facebook ad campaign, aimed specifically at cluster-headache sufferers in Oregon, or maybe just in Portland to recruit for a specific project/business.)
Are there any nations where DMT or other tryptamines are legal? Surely in Peru or Brazil, which allow ayahuasca? Building up the evidence base with studies in those countries (and maybe even rolling it out as a standard treatment for cluster headaches there) might help to accelerate the process of approval here in the USA and other nations.
How different does a “DMT vape pen” have to be from existing vaping devices? Could this become a grey-market smoke-shop-style product in the USA, where some renegade scientist-entrepreneur sells you everything you need except the psychedelics? (Not sure if this would be a good or bad thing to try, wouldn’t want to provoke regulators’ wrath and set the field back… but maybe with more thought this idea could turn into a more promising plan.)